Page 22 - Haematologica May 2022
P. 22
G. Escherich et al.
Outcome of randomized groups
No significant differences in outcome regarding EFS and OS were observed between the randomized arms (Figure 3C and D), with a median observation time of 3.7 years. There were also no significant differences in Cox regression analyses regarding the covariates sex, age (<10 years vs. ≥10 years), WBC (< 25/nL vs. ≥25/nL), ETV6-RUNX1, and HSCT in first continuous remission as time-dependent variables. An additional stratified analysis confirmed that there were no significant differ- ences in EFS or relapse rate between randomized cours- es according to the categories negative, positive non- quantifiable (n.q.), and quantifiable MRD on day 50.
Besides events that were anticipated upon quantifiable MRD after randomized intervention, several relapses occurred in MRD-negative and MRD-positive n.q. patients in both randomized treatment arms, accounting for the observed lack of surrogacy of MRD in the out- come analysis (Online Supplementary Table S4). There was no evidence of a mutual impact between the ran- domizations at early consolidation and delayed intensi- fication in this study, as shown by very similar pEFS in the latter randomized arms (log-rank test P=0.88 for patients receiving doxorubicin and log-rank test P=0.50 for patients receiving daunorubicin during delayed intensification).
Table 2. Minimal residual disease response toward clofarabine/PEG-ASP versus high-dose cytarabine/PEG-ASP.
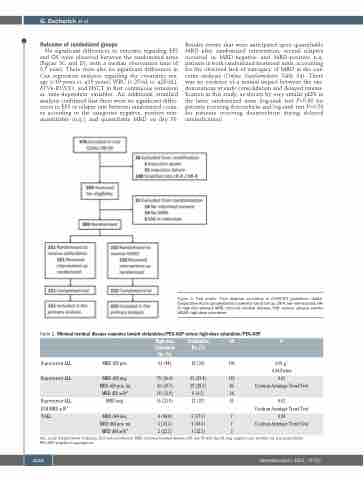
Figure 2. Trial profile. Flow diagram according to CONSORT guidelines. CoALL: Cooperative Acute Lymphoblastic Leukemia Study Group; LR-R: low risk-reduced; HR- R: high risk-reduced; MRD: minimal residual disease; SAE: serious adverse events; HIDAC: high-dose cytarabine.
B-precursor ALL B-precursor ALL
B-precursor ALL EOI MRD ≥10-3 T-ALL
MRD d50 pos.
MRD d50 neg. MRD d50 pos. nq MRD d50 ≥10-4 MRD neg.
MRD d64 neg. MRD d64 pos. nq MRD d64 ≥10-4
High-dose Cytarabine No. (%)
61 (44)
79 (56.4) 43 (30.7) 18 (12.9) 16 (21.9)
4 (44.4) 3 (33.3) 2 (22.2)
Clofarabine No. (%)
45 (33)
93 (67.4) 39 (28.3)
All P
106 0.03 c2 0.04 Fisher
172 0.01
82 Cochran-Armitage Trend Test
6 (4.3) 24
27 (37)
43 0.02 Cochran-Armitage Trend Test
3 (37.5)
4 (50.0)
1 (12.5) 3
7 0.94
7 Cochran-Armitage Trend Test
ALL: acute lymphoblastic leukemia; EOI: end-of-induction; MRD: minimal residual disease, d50: day 50; d64: day 64; neg: negative; pos: positive; nq: non-quantifiable. PEG-ASP: pegylated asparaginase.
1030
haematologica | 2022; 107(5)