Page 106 - Haematologica May 2022
P. 106
R. Ram et al.
Apheresis
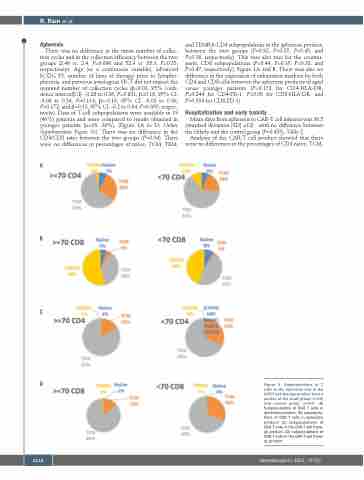
There was no difference in the mean number of collec- tion cycles and in the collection efficiency between the two groups (2.46 vs. 2.4, P=0.696 and 52.9 vs. 55.3, P=0.53, respectively). Age (as a continuous variable), advanced ECOG PS, number of lines of therapy prior to lympho- pheresis, and previous autologous HCT did not impact the required number of collection cycles (β=0.03, 95% confi- dence interval[CI]: -0.28 to 0.36, P=0.801; β=0.18, 95% CI: -0.06 to 0.54, P=0.114; β=-0.16, 95% CI: -0.02 to 0.36, P=0.172; and β=0.13, 95% CI: -0.2 to 0.64, P=0.305, respec- tively). Data of T-cell subpopulations were available in 19 (46%) patients and were compared to results obtained in younger patients (n=16, 39%), (Figure 1A to D; Online Supplementary Figure S1). There was no difference in the CD4/CD8 ratio between the two groups (P=0.94). There were no differences in percentages of naïve, TCM, TEM,
A
B
C
D
and TEMRA-CD4 subpopulations in the apheresis product, between the two groups (P=0.92, P=0.35, P=0.45, and P=0.16, respectively). This was also true for the counter- parts, CD8 subpopulations (P=0.44, P=0.35, P=0.33, and P=0.47, respectively), Figure 1A and B. There was also no difference in the expression of exhaustion markers by both CD4 and CD8 cells between the apheresis products of aged versus younger patients (P=0.172 for CD4-HLA-DR, P=0.244 for CD4-PD-1, P=0.06 for CD8-HLA-DR, and P=0.354 for CD8-PD-1).
Hospitalization and early toxicity
Mean days from apheresis to CAR-T cell infusion was 36.5 (standard deviation [SD] ±12) , with no difference between the elderly and the control group (P=0.453), Table 2.
Analysis of the CAR-T cell product showed that there were no differences in the percentages of CD4 naive, TCM,
Figure 1. Subpopulations of T cells in the apheresis and in the CAR-T cell therapy product from a portion of the study group (n=19) and control group (n=16). (A) Subpopulations of CD4 T cells in apheresis product; (B) subpopula- tions of CD8 T cells in apheresis product; (C) subpopulations of CD4 T cells in the CAR-T cell thera- py product; (D) subpopulations of CD8 T cells in the CAR-T cell thera- py product.
1114
haematologica | 2022; 107(5)