Page 218 - 2022_02-Haematologica-web
P. 218
Letters to the Editor
AB
CD
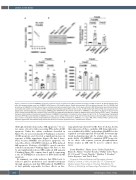
Figure 3. Interferon α-induced SAMHD1 expression regulates human megakaryocyte (MK) proplatelet formation and MK apoptosis. (A) Plot of Pearson corre- lation (R) between platelet count and SAMHD1 mRNA levels in 154 healthy donors. Dotted lines represent 95% confidence intervals. (B to D) CRISPR/Cas9 knock-down of SAMHD1 in CD34+ derived human umbilical cord cells were performed at day 3. Cells were cultured in MK differentiation promoting conditions, and treated with 1,000 U/mL interferon α (INFα) and phosphate-buffered saline (PBS) control. On day 13, MK treated with IFNa or PBS were assayed as men- tioned below. (B) Representative immunoblot of SAMHD1 after CRISPR-Cas9 knock-down (denoted as “SAMHD1 cr” throughout the figure) in day 13 human CD34+ derived cultured MK with or without IFNa. Guide RNA not targeting known genes are used as negative control with or without IFNa (“neg cr” throughout the figure). Fold changes of densitometric quantification of SAMHD1 immunoblots, normalized to actin is shown on right (n=5). (C) The percentage of proplatelet formation (PPF) MK, scored blinded, for SAMHD1 cr vs neg cr, with or without IFNa is shown (n=6). (D) The mean fluorescence intensity (MFI) of annexin V bind- ing (a marker of apoptosis-induced phosphatidylserine expression) on IFNa stimulation in SAMHD1 cr vs. neg cr MK, with or without IFNa is plotted (n=5). Statistical significance was determined by paired t-test (B to D). Error bars represent mean ± standard error of mean.
Although viral infections induce MK apoptosis,15 we are not aware of in vitro studies assessing IFNa-induced MK apoptosis. Under the culture conditions described in Figure 3C, we assessed annexin V binding as a measure of MK apoptosis, and observed a significant increase in response to IFNa stimulation (Figure 3D, first 2 bars). Next, because SAMHD1 also promotes apoptosis,9 we tested the effects of SAMHD1 deletion on IFNa-induced MK apoptosis. Deletion of SAMHD1 caused a modest reduction in annexin V binding (Figure 3D, bar 1 vs. bar 3), and significantly reduced IFNa-induced MK annexin V binding (Figure 3D, compare bars 2 and 4), supporting a role for SAMHD1 as a mediator of IFNa-induced MK apoptosis.
In summary, our study indicates that IFNa leads to reduced platelet production and thrombocytopenia through apoptosis, and that IFNa-induced SAMHD1 is at least partially responsible for these effects on late-
stage platelet production by MK. Prior work has shown that expression of three candidate MK transcription fac- tors is inhibited by IFNa4, and perhaps SAMHD1 is also regulated at a transcriptional level in MK. Post-transcrip- tional mechanisms may also be at play, since the enzy- matic ability of SAMHD1 to maintain dNTP homeosta- sis in other cells requires protein phosphorylation. Future studies in MK will be need to address these issues.
Seema Bhatlekar,1 Shancy Jacob,1 Emilia Tugolukova,1 Bhanu K. Manne,1 Yasuhiro Kosaka,1 Phillipe Loher,2 Ryan M. O’Connell,3 Vicente Planelles,4 Matthew T. Rondina,1,5 Isidore Rigoutsos5 and Paul F. Bray1,6
1Program in Molecular Medicine and Department of Internal Medicine, University of Utah, Salt Lake City, UT; 2Computational Medicine Center, Thomas Jefferson University, Philadelphia, PA; 3Division of Microbiology and Immunology, Department of Pathology,
560
haematologica | 2022; 107(2)