Page 216 - 2022_02-Haematologica-web
P. 216
Letters to the Editor
Interferon a-induced SAMHD1 regulates human cultured megakaryocyte apoptosis and proplatelet formation
Megakaryocyte (MK) growth, differentiation and mat- uration are required for thrombopoiesis and platelet pro- duction. Most studies of megakaryocytopoiesis have uti- lized in vitro culture systems expected to model a healthy human condition. However, consistent with the ability of MK to respond to inflammatory mediators, chronic inflammatory conditions often induce thrombocytosis, whereas acute inflammation can result in thrombocy- topenia. Furthermore, there is an increasing awareness of the role MK play in innate and adaptive immunity.1 Type 1 interferons (IFN-1), including IFNa, IFNb and INFω are a family of cytokines that bind to the IFN-1 receptor and trigger transcription of diverse genes. IFN- inducible genes regulate resistance to viral infections, enhance innate and adaptive immunity, and modulate
normal and tumor cell survival and death.2 MK express the IFN-1 receptor that signals through Janus kinases/signal transducer and activator of transcription proteins (JAK/STAT) pathway in response to IFN-1 cytokines.3 IFNa, an IFN-1 cytokine, has been effectively utilized in the treatment of myeloproliferative neo- plasms and viral hepatitis. Thrombocytopenia is a com- mon adverse effect of IFNa therapy that can require dose reduction. Although there are inconsistent reports regarding IFNa suppression of colony forming units of megakaryocyte progenitors (CFU-MK) in cultures of human CD34+ cells, there are consistent findings to sup- port a mechanism of decreased platelet production rather than reduced platelet life span.4-7 However, the molecular mechanisms regulating IFNa-induced decrease in platelet production and peripheral blood thrombocytopenia are poorly understood. The major conclusions in this report are (i) using genome-wide gene expression profiling we show that IFNa upregulates the
ABC
DEF
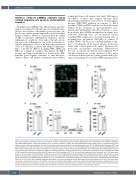
Figure 1. Interferona regulates platelet production. (A to E) CD34+ hematopoietic stem cells and progenitor cells were isolated by immunomagnetic separation from human umbilical cord blood. Cells were cultured for 13 days in stem cells expansion media supplemented with thrombopoietin that promotes megakary- ocyte (MK) differentiation. 1,000 units/milliliter (U/mL) of human interferon α (INFα) and phosphate-buffered saline (PBS) (used as a negative control) was added at day 9 and further incubated until day 13. All assays mentioned in panels A to E were performed at day 13. (A) MK proplatelet formation (PPF) was counted blinded as to the IFNa treatment. At least 200 cells were counted per culture (n=4). (B) Representative images of day 13 cultured MK treated with PBS or IFNa. Treated MK were plated on fibrinogen on day 12 overnight, and fixed with 4% paraformaldehyde, stained with Alexa Fluor 488 Phalloidin (green) and a nuclear stain, DAPI (blue). Images were taken by a confocal microscope at 40X oil objective lens. (C) Platelet-like particles (PLP) were collected from IFNa or PBS-treated MK cultures and stained with APC labeled anti-CD41a antibody at 37°C for 10 minute and measured by flow cytometry (n=3). PLP were gated based on human peripheral blood platelets. (D) MK were stained with APC-labeled CD41a and PE-labeled CD42a antibodies, and CD41a+ CD42a+ MK (a marker for MK maturation) were assessed by flow cytometry (n=3). (E) Cultured MK treated with IFNa or PBS were stained with APC-labeled CD41a and propidium iodide, and ploidy was assessed by flow cytometry (n=3). The quantification of the ploidy distribution is shown on the y-axis by calculating the percentage of cells with 2n, 4n, 8n and 16n. Apoptotic population were gated out. Statistical significance was determined by two-tailed paired t-test (A to E). Error bars repre- sent mean ± standard error of mean. (F) 25,000 units of murine IFNa or PBS (negative control) were administered intraperitoneally in wild-type mice for con- secutive three days (n=5 per group). On day 4, mice blood was harvested by cardiac puncture and platelet count was measured by Hemavet. Statistical signifi- cance was determined by two-tailed unpaired t-test with Welch’s correction. Error bars represent mean ± standard error of mean.
558
haematologica | 2022; 107(2)