Page 260 - 2022_01-Haematologica-web
P. 260
A.O. Khan et al.
FG
H
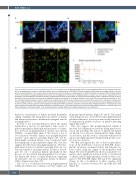
Figure 4. Platelet activation results in polyglutamylation of the marginal band. (A) Resting platelets show a partial polyglutamylation of the marginal band and a loss of the polyglycylation evident in induced pluripotent stem cell megacaryocytes (iPSC-MK). (B) Platelet spreading on fibrinogen and collagen shows an accumu- lation of polyglutamylation at the marginal band as platelets spread, and no evidence of polyglycylation. (C) Western blotting of resting and CRP activated platelets confirms the presence of polyglutamylated tubulin and a loss of polyglyclation. No increase in polyglutamylation is evident over time. (D, E) A measurement of co- localization (corrected Manders coefficient) between polyglutamylated tubulin and b1-tubulin in resting and spread platelets shows a significant increase in the co- localization of these modified tubulin residues on platelet activation and spreading. (F, G) Platelets activated in vitro using CRP were fixed and co-stained for b1-tubu- lin and polyglutamylated residues, and then imaged in 3D using AiryScan confocal (stacks colorized in Z as indicated by the color chart in this figure). In these micro- thrombi, polyglutamylation of the marginal band is evident, while polyglycylation is not observed. (H) A time course was performed to compare polyglutamylated tubu- lin with two other previously reported post-translational modifications (PTM) in platelets (acetylation and tyrosination). Polyglutamylation is maintained over time, while acetylation and tyrosination decrease significantly as platelets spread. (I) The mean fluorescence intensity of polyglutamylated tubulin is markedly higher than either acetylated or tyrosinated tubulin. (n=3, standard deviation. Two-Way ANOVA with multiple comparisons. 10 mm scale bar.)
I
driven by a mechanism of dynein mediated proplatelet sliding.33 Similarly, the antagonistic movement of dynein and kinesin are known to maintain the marginal band in resting platelets.13
In order to test if polymodification affects the spatial distribution of motors, we performed a time course of platelet spreading on fibrinogen and measured co-localiza- tion between polyglutamylated tubulin and dynein (DNAL1 - axonemal light chain 1). We observe a loss of co-localization between dynein and polyglutamylated residues upon platelet spreading (Figure 5A and B). Interestingly, axonemal dynein is also localized towards the leading edge of spread platelets (Figure 5A). This data suggests that the increased polyglutamylation of the mar- ginal band observed on platelet spreading drives an out- ward movement of axonemal dynein. In order to investi- gate the role of axonemal dynein specifically in this process, we also stained spreading platelets for cytoplas- mic dynein and observe a central distribution, suggesting an alternative role for cytoplasmic dynein in platelets (Online Supplementary Figure S6).
The loss of co-localization between polyglutamylated tubulin and dynein was confirmed on both collagen and
fibrinogen spread platelets (Figure 5C and E). This spatial relationship was also observed between polyglutamylated tubulin and kinesin-1, a motor protein recently reported to be important in platelet secretion on platelet spreading (Figure 5D and F).34
This data suggests that polyglutamylated tubulin is involved in localizing motor proteins during platelet acti- vation and spreading. The actions of kinesin and dynein are known to be critical to driving platelet shape change on activation, and this work is consistent with previous reports of polyglutamylated tubulin altering the processiv- ity of motors in axons.
We then investigated the role of these polymodifica- tions on the distribution of motors in iPSC-MK. In pro- platelet extensions axonemal dynein and kinesin-1 are both evident along the length of the proplatelet shaft (Figure 5G). b1-tubulin KO cells show no proplatelet for- mation, and a significant reduction in the co-localization of dynein with polyglutamyulated residues when com- pared to WT iPSC-MK (Figure 5H and I). No significant change in the co-localization of these residues with kinesin-1 is observed between WT and KO iPSC-MK (Figure 5J).
252
haematologica | 2022; 107(1)