Page 183 - 2021_12-Haematologica-web
P. 183
Letters to the Editor
his parents identified a de novo heterozygous nonsense mutation (c. 6037C>T p.Q2013X) in the SPTB gene. This gene encodes a member of the spectrin family, involved in the stability of erythrocytes membranes. Mutations in this gene have been associated with heredi- tary spherocytosis and hemolytic anemia.5 While most symptoms of the proband were consistent with hereditary spherocytosis, the non-regenerative anemia, together with the presence of ring sideroblasts and extra-hematological signs remained unexplained, suggesting that additional mutations were involved in his phenotype. According to this hypothesis, we identified in the proband two com- pound heterozygous missense variants (c.1031 G>A p.R344Q and c.1205 G>A p.R402H) in the SARS2 gene (cDNA accession number: NM_017827.4, transcript ID: ENST00000221431), each inherited from one of two par- ents. SARS2 is an ubiquitously expressed nuclear gene encoding a mitochondrial aminoacyl-tRNA synthetase (mt- aaRS) whose function is to catalyze the specific ligation of serine to two mitochondrial tRNA isoacceptors: tRNASer(AGY) and tRNASer(UCN).6 Both SARS2 variants were reported to be rare in public human genetic variant data- bases (GnomAD_exomes MAF: T=0.000012 and T=0.000008 respectively). As they affect highly conserved amino acid residues located in the core catalytic domain of the protein and are predicted to be damaging by in silico softwares, biallelic SARS2 mutations identified in the proband were very likely to result in loss-of function.
SangervalidationofthemutationsinSPTBandSARS2are shown in the Online Supplementary Figure S1C and Figure 1C.
Missense mutations in the SARS2 gene (p.V223M, p.D390G and p.R402H) have been reported so far in six individuals from four distinct families who presented a spe- cific MID characterized by hyperuricemia, pulmonary hypertension, renal failure in infancy and alkalosis and des- ignated as HUPRA syndrome.7–9 Another SARS2 variant (homozygous splicing mutation in exon 14) was reported in a single case suffering from progressive spastic paresis instead of HUPRA syndrome,10 in agreement with the known phenotypic heterogeneity of mutations involving mitochondrial machinery.
Interestingly, a careful review of the published cases of HUPRA syndrome revealed that five of six were anemic with a Hb level varying from 4.8 to 9 g/dL (Online Supplementary Table S2). However, it appears that anemia associated with HUPRA syndrome has never been investi- gated mechanistically. We therefore hypothesized that SARS2 plays an important role in erythropoiesis. As all SARS2 variants associated with HUPRA syndrome were expectedtoresultinloss-offunction,7–9weusedaninvitro SARS2 knockdown model to explore the consequences of SARS2 inactivation on erythropoiesis.
In a widely used primary cell culture system that recapit- ulates erythropoiesis, CD34+ were isolated from unrelated cord blood and expanded for 5 days in a medium contain-
A
BC
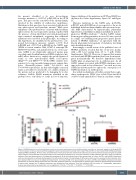
Figure 2. SARS2 depletion in early erythroblasts results in decreased proliferation rate and differentiation arrest due to increased apoptosis. (A) At day 5 (D5) of the first phase of culture, primary erythroid progenitors were transduced with scramble short hairpin RNA (shCTRL) or short hairpin RNA (shRNA) specifically targeting SARS2 (shSARS2). 48 hours later, cell proliferation of transduced cells was monitored for 150 hours by real-time videomicroscopy using the Incucyte® system. Results are represented as the fold increase of cell confluence normalized to T0. Error bars represent standard deviation (SD) from mean of 3 technical replicates. Data are representative of 3 independent experiments. (B) At the indicated days of the second phase of culture, shCTRL- and shSARS2-transduced cells were stained with an antibody directed against the GPA erythroid marker. The percentage of GPA positive cells was then assessed by flow cytometry. Error bars represent SD from mean of 3 independent experiments. (C) At day 6 (D6) of the second phase of culture, shCTRL- and shSARS2-transduced cells were stained with an antibody directed against Annexin V and with propidium iodide (PI). The percentage of apoptotic cells, defined as the percentage of the Annexin V positive cells amongst the PI negative population was assessed by flow cytometry. Error bars represent SD from mean of 3 independent experiments. P-values are determined by two-tailed t-test. ns: not significant; *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
haematologica | 2021; 106(12)
3203