Page 182 - 2021_12-Haematologica-web
P. 182
Letters to the Editor
Biallelic mutations in the SARS2 gene presenting as congenital sideroblastic anemia
Mitochondrial disorders (MID) are a clinically heteroge- neous group of metabolic disorders characterized by impaired mitochondrial function. They can be caused by mutations in either mitochondrial DNA (mtDNA) or nuclear DNA encoding mitochondrial proteins. In addition to energy deficiency, consequences of mitochondrial dys- function can include excessive reactive oxygen species (ROS) production, defect in heme synthesis, aberrant calci- um handling, and apoptosis dysregulation, all of which contribute to the pathogenesis of MID.1 MID are usually multisystemic disorders, affecting various organs but only a few of them such as congenital sideroblastic anemias (CSA) display major erythroid abnormalities. CSA is a series of rare, heterogeneous disorders characterized by pathological iron accumulation in the mitochondria of erythroblasts and presence of ring sideroblasts in the bone marrow at varying degrees.2,3 So far, nearly two-thirds of CSA cases have been attributed to a mutation in a specific gene or genes.4
Here we report the original case of a patient presenting with CSA, in which we identified biallelic mutations of the seryl-tRNA synthetase 2 (SARS2) gene.
The affected child was a first-born son to a non-consan- guineous healthy couple with no neonatal antecedents and no familial medical history. His younger sister is healthy (Figure 1A). He presented at the age of 3 years with chronic
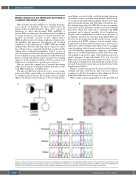
renal failure associated with cerebellar atrophy, hyperten- sion, failure to thrive, polyuria and polydipsia. Kidney biop- sy revealed a tubulointerstitial nephritis. Metabolic workup showed hyperlactatemia and abnormal acylcarnitine pro- file, findings suggesting of a MID. Blood tests concomitant- ly revealed a non-regenerative anemia associated with mild hemolysis, which was refractory to erythropoietin (EPO) treatment and required monthly blood transfusions. Negative direct antiglobulin test ruled out the presence of an immune disorder. No abnormal hemoglobin (Hb) was detected on electrophoresis profile and the levels of pyru- vate kinase and G6PD enzymes were in normal values. Peripheral blood smear showed the presence of about 20% spherocytes (Online Supplementary Figure S1A). Accordingly, osmotic gradient ektacytometry revealed reduced erythro- cyte deformability, supporting the diagnosis of hereditary spherocytosis (Online Supplementary Figure S1B). Bone mar- row aspiration showed no signs of dyserythropoiesis but a small contingent of ring sideroblasts (5%) was observed (Figure 1B). Iron and transferrin serum levels were in nor- mal ranges but ferritin level and transferrin saturation were elevated. Patient’s laboratory data are detailed in the Online Supplementary Table S1.
Patient’s condition progressively deteriorated leading to terminal end-stage renal disease and very severe anemia requiring weekly blood transfusions. He ultimately died of pulmonary hypertension at the age of 5 years.
Whole exome sequencing performed on the proband and
AB
C
Figure 1. Clinical and molecular findings of the proband. (A) Pedigree and segregation of the pathogenic SARS2 variants in the reported family. (B) Bone marrow aspiration from the proband showing the presence of ring sideroblasts on iron stain. (C) Sanger sequencing validation of SARS2 c.1031 G>A (p.R344Q) and c.1205 G>A (p.R402H) mutations detected by next generation sequencing. Red arrows indicate the position of the nucleotide’s substitution.
3202
haematologica | 2021; 106(12)