Page 32 - 2021_09-Haematologica-web
P. 32
R. Svanberg et al.
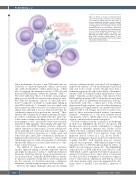
Figure 4. Effects of immune checkpoint block- ade, chimeric antigen receptor T cells, and bispe- cific antibodies. Arrows point out the actions of immune checkpoint blockade, chimeric antigen receptor T cells and bispecific antibodies, leading to engagement and activation of the tumor microenvironment for anti-chronic lymphocytic leukemia activity. Upward arrows indicate increas- es. CLL: chronic lymphocytic leukemia; PD-1: pro- grammed cell death protein 1; PD-1L: pro- grammed cell death protein-1 ligand; Ab: anti- body; CAR-T: chimeric antigen receptor T cells; bsAB: bispecific antibodies; TCR: T-cell receptor; CD: cluster of differentiation.
Major mechanisms of action of anti-CD20 antibodies are activation of antibody-dependent cellular cytotoxicity and antibody-dependent cellular phagocytosis, which rely on engaging the antitumor activity of NK cells and monocytes/macrophages within the immune TME.1,48,93 The direct inhibitory effects of ibrutinib on macrophage phagocytosis and NK-cell activation may therefore inter- fere with the therapeutic efficacy of anti-CD20 treat- ment.48 Compared to ibrutinib as a single agent, adding an anti-CD20 antibody to ibrutinib was associated with faster remissions and lower levels of residual disease in a clinical trial, although it was not demonstrated that the combination improved progression-free survival.94 Thus, whether this combination is beneficial remains debatable. In contrast, combining anti-CD20 antibodies with vene- toclax seems to improve the phagocytosis of CLL cells by macrophages in vitro,93 and reverse venetoclax resistance induced by TME signaling.71 Interestingly, although vene- toclax plus anti-CD20 treatment produces impressive clinical responses in clinical trials, a recent retrospective study including real-world data demonstrated compara- ble efficacy between venetoclax as a single agent and venetoclax plus anti-CD20 combination treatment in high risk relapsed/refractory CLL patients.95 Thus, further validating prospective studies are warranted to determine whether the addition of an anti-CD20 antibody to vene- toclax is truly necessary.
The addition of venetoclax to ibrutinib constitutes another approach aiming to provide improved duration and depth of remissions as well as to enable fixed-duration treatment, which has already, in part, demonstrated suc- cess in clinical trials.96 Similarly, the PI3K inhibitor, duvelis- ib, increases sensitivity of CLL cells to venetoclax, provid- ing the rationale for duvelisib-venetoclax combination treatment, currently being investigated in clinical trials.97
However, the biggest challenges ahead involve finding
strategic combinations that overcome T-cell dysfunction, improve the efficacy of T-cell-based therapies and ICB in CLL, and work towards curative therapy. Data from a human xenograft model support the ability of ibrutinib to enhance CAR T-cell function when administered concur- rently.98 Similarly, another murine study indicated that PI3Kδ inhibition during CAR T-cell production may have a positive effect on engraftment and antitumor activity.99 Consistently with this, a clinical pilot study recently demonstrated high response rates in relapsed/refractory CLL patients receiving ibrutinib concomitant with CD19- targeted CAR T-cell therapy, and lower toxicities com- pared to those in patients treated without concomitant ibrutinib.100 Furthermore, T cells from ibrutinib-treated CLL patients seem to exhibit improved in vitro anti-CLL activity combined with bispecific antibodies.101
The lack of clinical activity of anti-PD-1 monotherapy in CLL,84 has highlighted the need to incorporate ICB therapies into more powerful combinations to unleash the power of antitumor immune cells. Studies of PD- 1:PD-L1 blockade combined with ibrutinib have demon- strated enhanced CD8+ T-cell function along with improved disease control in a CLL mouse model.102 However, preliminary clinical results have indicated that coupling anti-PD-1 with ibrutinib may not increase response rates in patients.103 PI3K inhibition improved the anticancer effect of ICB through modulatory effects on MDSC in a solid cancer in vitro model,104 thus highlighting additional roles of PI3K inhibition in modulation of the TME which could be exploited. The relative expansion of memory T-cell subsets due to direct effects of venetoclax on other, more BCL-2-dependent T-cell subsets, support a role for venetoclax in combination with ICB. In a recent sold cancer murine study, venetoclax augmented the anti- tumor effect of anti-PD-1 and anti-PD-L1 inhibitors in vivo.69
2320
haematologica | 2021; 106(9)