Page 31 - 2021_09-Haematologica-web
P. 31
Targeting the tumor microenvironment in CLL
recent study revealed that CAR T cells from CLL patients responding well to CAR T-cell therapy expressed upregu- lated genes associated with T-cell memory. Furthermore, enriched T-memory subsets prior to CAR T-cell genera- tion correlated with sustained remissions. Meanwhile, CAR T cells from non-responders had upregulated genes associated with effector T-cell differentiation, apoptosis and exhaustion, thus emphasizing that T-cell fitness is crucial for the efficacy of CAR T cells.89 Due to the multi- tude of (successful) treatment options for CLL currently, CAR T-cell therapy may first become a relevant option in treating multi-relapsed disease, and preliminary reports from current clinical trials of CD19-targeted CAR T-cell therapy in patients with multi-relapsed CLL show some- what encouraging results.90 However, paradoxically, T- cell exhaustion in CLL is demonstrated to worsen with progressive disease,13 thus pointing towards a need for options that improve T-cell function prior to the manu- facture of CAR T cells or during treatment. Furthermore, it was recently elucidated that CLL cells can directly impair CAR T-cell function and induce an exhausted phe- notype through the release of plasma extracellular vesi- cles.91 Thus, a meaningful role for CAR T-cell therapy in CLL may rely on the ability of current and/or future ther- apies to successfully target the TME and improve T-cell fitness in patients with CLL, prior to the CAR T-cell treat- ment, during preparation of the product, and after its administration.
A novel therapeutic approach that could constitute an alternative to CAR T-cell therapy is off-the-shelf bispecif- ic CD19/CD3 or CD20/CD3 antibody treatment.
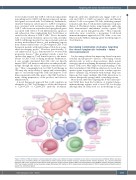
Bispecific antibodies simultaneously engage CD3 on T cells and CD19 or CD20 on target B cells, and thereby redirect T cells to recognize CLL cells, facilitating synapse formation and, thus, T-cell-mediated antitumor responses (Figure 4). Preclinical studies using bispecific antibodies have demonstrated antileukemic activity against CLL cells in vitro and in xenograft models.92 Thus, bispecific antibodies may constitute a promising T-cell-based immunotherapeutic approach for CLL, alone, or in com- bination with TME-modulating agents that help improve T-cell function.
Developing combination strategies targeting the chronic lymphocytic leukemia – tumor microenvironment
It is becoming evident that improving clinical responses (residual and progressive disease), overcoming toxicity, infection risk, as well as drug resistance, likely require strategies aimed at reshaping the immunosubversive, pro- tumor TME state. Our improved understanding of the direct and indirect CLL-TME modulations by novel ther- apeutic agents in recent years provides a unique opportu- nity to optimize CLL treatment with strategic drug com- binations that target multiple CLL-TME interactions to achieve therapeutic synergy while controlling toxicity.
Monoclonal antibodies targeting the B-cell surface pro- tein CD20 have been the backbone of standard chemo- immunotherapy regimes used to treat CLL for decades, although they are rarely used as a monotherapy in CLL.1
Figure 3. Effects of BCL-2 inhibitors, immunomodulatory drugs, and cereblon E3 ligase modulation on the tumor microenvironment. Inhibitory effects are repre- sented by bars, stimulatory effects are represented by arrows. Upward arrows indicate increases, downward arrows indicate decreases. CLL: chronic lymphocytic leukemia; TME: tumor microenvironment; BCL-2: B-cell lymphoma 2; BCL-2i: BCL-2 inhibitor; IMiD: immunomodulatory drug; CELMoD: cereblon E3 ligase modulator; TCR: T-cell receptor; HLA-DR: human leukocyte antigen DR-isotype; IFN: interferon; PD-1: programmed cell death protein 1; PD-1L: programmed cell death protein-1 ligand; CCL: chemokine ligand.
haematologica | 2021; 106(9)
2319