Page 137 - 2021_06-Haematologica-web
P. 137
InHIBIT-Bleed: bevacizumab for bleeding in HHT
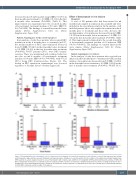
decreased from 6.0 (interquartile range [IQR], 0.0-13.0) in the 6 months pretreatment to 0.0 (IQR, 0.0-1.0) in the first 6 months after treatment (P<0.0001) (Table 2). This improvement was maintained into the second 6 months of bevacizumab treatment (median of 0.0 units, IQR 0.0- 0.0; P=0.0005). The findings of a mixed linear model were similar (Online Supplementary Table S2, Online Supplementary Figure S1C).
Patients requiring prior red blood cell transfusion
In an analysis of only those patients who required RBC transfusion in the 6 months prior to treatment (n=137), the median number of RBC units transfused decreased from 9.0 (IQR, 5.0-16.0) in the 6 months before treatment to 0.0 (IQR, 0.0-2.0) in the first 6 months after treatment (P<0.0001); 80/137 patients (58%), were RBC transfu- sion-free. There was maintained and continued reduction into the second 6 months of bevacizumab treatment (median of 0.0 units, IQR 0.0-0.0; P=0.0005), with 97/121 (80%) being RBC transfusion-free (Figure 1C). The decline in RBC transfusion requirements was observed regardless of baseline disease severity (Figure 2A).
Effect of bevacizumab on iron infusion
All patients
A total of 183 patients who had been treated for ≥6 months had complete iron infusion data available and were included in the iron infusion analysis. In the analysis of all included patients (those who required iron infusion in the 6 months prior to treatment and those who did not), the median number of iron infusions decreased from 6.0 (IQR, 1.0-18.0) in the 6 months before treatment to 1.0 (IQR, 0.0- 4.0) in the first 6 months after treatment (P<0.0001) (Table 2). This improvement continued into the second 6 months of bevacizumab treatment (median of 0.0 infusions, IQR 0.0-2.0; P<0.0001). The findings of a mixed linear model were similar (Online Supplementary Table S2, Online Supplementary Figure S1D).
Patients requiring prior iron infusion
In the analysis of only those patients who received iron infusion in the 6 months prior to treatment (n=155), median number of iron infusions decreased from 8.0 (IQR, 3.0-20.0) in the 6 months before treatment to 2.0 (IQR, 0.0-5.0) in the first 6 months after treatment (P<0.0001); 48/155 (31%)
AB
CD
Figure 1. Box-and-whisker plots showing the effect of intravenous bevacizumab on hematologic parameters and epistaxis severity. A box represents the median and interquartile range and tails represent minimum and maximum values. Numbers at each time-point reflect the number of hereditary hemorrhagic telangiectasia (HHT) patients in a given analysis treated for at least that duration with complete data. (A) Effect on hemoglobin in HHT patients with baseline anemia (n=185). (B) Effect on Epistaxis Severity Score in patients treated for epistaxis (n=146). (C) Effect on red blood cell (RBC) transfusions in patients receiving transfusion in the 6 months prior to bevacizumab initiation (n=137). (D) Effect on iron infusion events in patients receiving intravenous iron in the 6 months prior to bevacizumab initiation (n=155). RBC: red blood cell; Mo: months; Tx: treatment.
haematologica | 2021; 106(8)
2165