Page 189 - 2021_07-Haematologica-web
P. 189
Lenalidomide induction and maintenance in MM
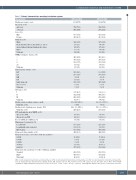
Table 1. Patients’ characteristics according to induction regimen.
Characteristic
Median age (range), years
CRD (n=1,021)
61 (28-75)
772 (75.6)
249 (24.4)
610 (59.7) 411 (40.3)
938 (91.9) 21 (2.1) 28 (2.7) 10 (0.9) 24 (2.4)
421 (41.2) 363 (35.6) 119 (11.7) 53 (5.2) 65 (6.4)
633 (62.0) 220 (21.5) 4 (0.4) 12 (1.2) 139 (13.6) 6 (0.6) 7 (0.7)
301 (29.5) 392 (38.4) 246 (24.1)
82 (8.0) 85.0 (28.0-825.0) 9 (8.8)
262.0 (3.0-2519.0) 228 (22.3)
85 (8.3)
82 (8.0) 35 (3.4)
230 (22.5) 103 (10.1) 190 (18.6) 414 (40.5)
56 (13.5) 8 (1.9) 3 (0.7) 31 (7.5) 137 (33.1)
223 (53.9) 149 (36.0) 42 (10.1)
CTD (n=1,021)
61 (29-74)
754 (73.8)
267 (26.2)
611 (59.8) 410 (40.2)
937 (91.8) 14 (1.4) 27 (2.6) 14 (1.4) 29 (2.8)
439 (43.0) 367 (35.9) 135 (13.2) 34 (3.3) 46 (4.5)
600 (58.8) 269 (26.3) 4 (0.4) 9 (0.9) 127 (12.4) 7 (0.7) 5 (0.5)
306 (30.0) 388 (38.0) 253 (24.8)
74 (7.2) 83.0 (30.0-897.0) 7 (6.9)
273.0 (0.0-3550.0) 215 (21.1)
98 (9.6)
102 (10.0) 38 (3.7)
221 (21.6) 93 (9.1) 187 (18.3) 422 (41.3)
70 (16.6) 12 (2.8) 2 (0.5) 42 (10.0) 136 (32.2)
236 (55.9) 117 (27.7) 69 (16.4)
Age group, n (%)
≤65 years
>65 years Sex, n (%)
Male
Female
Ethnicity, n (%)
White
Black (Black Caribbean, Black African, other) Asian (Indian, Pakistani, Bangladeshi, other) Other
Unknown
WHO performance status, n (%) 0
1
2
≥3 Unknown
Immunoglobin subtype, n (%)
IgG
IgA
IgM
IgD
Light chain only Non-secretor Unknown
ISS stage, n (%) I
II
III Unknown
Median serum creatinine (range), mol/L
Unknown, n (%)
Median lactate dehydrogenase (range), IU/L
Unknown, n (%)
CVD randomization after MR/PR, n (%)
Allocated to CVD
Allocated to no CVD
Received CVD after SD/PD, n (%) Maintenance treatment, n (%)
Lenalidomide
Lenalidomide plus vorinostat Observation
Cytogenetic data available, n (%)
Cytogenetic lesions, n (% of those with data available)
t(4;14) t(14;16) t(14;20) del(17p) gain(1q)
Cytogenetic risk category, n (% of those with data available) Standard
High*
Ultra-high†
CRD: cyclophosphamide, lenalidomide, and dexamethasone; CTD: cyclophosphamide, thalidomide, and dexamethasone; CVD: cyclophosphamide, bortezomib, and dexametha- sone; Ig: immunoglobulin; ISS: International Staging System; MR: minimal response; PD: progressive disease; PR: partial response; SD: stable disease; WHO: World Health Organization. *High risk defined as the presence of any one of t(4;14), t(14;16), t(14;20), del(17p), or gain(1q). †Ultra-high risk defined as the presence of more than one lesion.
haematologica | 2021; 106(7)
1959