Page 94 - 2021_05-Haematologica-web
P. 94
L. Doll et al.
cells (Figure 4F). The last gene is expressed in monocytes and neutrophils. In contrast, mpeg1.1+ macrophages41 were not affected (Figure 4G). With regard to definitive hematopoiesis, no difference was observed in the expres- sion of the hematopoietic stem cell marker cmyb between wild-type and hax1 morphants at 2 dpf (Figure 4H). Together, these results indicate that hax1 is dispensable for embryonic erythropoiesis and monopoiesis.
Interference with Hax1 function enhances apoptosis in embryos but not in neutrophils
We next sought to determine the underlying molecular basis for the role of Hax1 in neutrophil development. To date, two main observations associated with HAX1 defi- ciency in CN patients are increased apoptosis of myeloid progenitors,6 and decreased activity of the G-CSF signal transduction pathway.18 Based on these findings, we examined to what extent cellular viability and the G-CSF signaling pathway were affected in hax1 morphants. We used three approaches to identify apoptotic cells. First, a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was performed to detect apoptot- ic cells. Compared to their wild-type counterparts, hax1 morphants exhibited increased apoptosis at 1 dpf (Figure 5A and B). The increased apoptosis at 1 dpf was specific to the hax1 knockdown fish because no significant differ-
ence was observed when a control MO was injected (Figure 5B). By 2 dpf, the number of apoptotic cells was comparable between morphants and uninjected embryos (Figure 5C, Online Supplementary Figure S6). As a second approach, we stained wild-type embryos and hax1 mor- phants with the fluorescent dye acridine orange (Figure 5D). Similar to the TUNEL assay, this showed that the number of apoptotic cells was increased in the hax1 mor- phants at 1 dpf (Figure 5E), but not at 2 dpf (Figure 5F). Strikingly, apoptotic cells stained with acridine orange as well as TUNEL were scattered throughout the embryo and not preferentially associated with sites of hematopoiesis. In support of this notion, we incubated wild-type and hax1-injected tg(lyz:dsRED) embryos with a caspase 3/7 reporter, which produces a green fluoro- genic response upon cleavage by activated caspase-3 or caspase-7. Confocal imaging of 2 dpf transgenic tg(lyz:dsRED) embryos (Figure 5G) showed that the fre- quency of dsRED+ cells stained with the caspase 3/7 reporter was comparable in the wild-type and hax1 mor- phants at 2 dpf (Figure 5H). It is also worth noting that interference with Hax1 function did not affect cell prolif- eration in embryos (Online Supplementary Figure S7). These findings, therefore, indicate that hax1 knockdown enhances apoptosis in zebrafish embryos at early stages, but not in neutrophils.
AB
CD
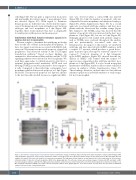
Figure 2. Knockdown of hax1 impairs neutrophil development. (A) Relative change of wild-type hax1 transcript in the hax1 morphants compared with wild-type (WT) using quantitative polymerase chain reaction. N indicates number of biological replicates. (B) Representative images of mpo-stained cells in WT and e1-MO injected embryos (left panel). Note that each stained cell represents a neutrophil. The right panel shows numbers of mpo stained cells in the trunk region at 24 hours post- fertilization (hpf). (C) Injection of hax1 morpholinos (MO) in the tg(mpo:gfp) line. The left panel shows representative images of uninjected (WT) and hax1 e1-MO injected transgenic embryos at 48 hpf. The right panel shows numbers of green fluorescent protein-positive cells in the trunk region. (D) Co-injection of e1-MO or e2- MO morpholinos with hax1 mRNA rescued the reduced neutrophil numbers in the tg(mpo:gfp) line. Scale bars indicate 100 mm. Each dot represents an individual embryo. Data are means ± standard deviation.
1314
haematologica | 2021; 106(5)