Page 225 - 2021_04-Haematologica-web
P. 225
Sec22b controls VWF trafficking and WPB size
Figure S2D). A similar reduction of WPB length was observed in endothelial cells targeted with three separate gRNA directed to exon 1 of Sec22b (Figure 1B, Online Supplementary Figure S2E). As a third, independent strategy we also determined WPB morphology after expression of an mEGFP-tagged non-fusogenic Sec22b variant (mEGFP- Sec22b-DSNARE), which lacks the SNARE domain responsible for fusion and compared these with mEGFP- expressing cells (Figure 1D). We found that the DSNARE construct had a dominant-negative effect on WPB size, with these WPB being significantly shorter than the mEGFP control (Figure 1E). To investigate whether the size reduction extends to other (post-Golgi) organelles, we determined the localization of the tetraspanin CD63. CD63 normally cycles between plasma membrane, endo- lysosomal organelles and WPB in an AP-3-dependent manner.37 Silencing of Sec22b did not lead to apparent changes in the morphology of CD63+ endolysosomal organelles, nor did it impede the trafficking of CD63 to shorter WPB (Online Supplementary Figure S3). Together these results identify Sec22b as a determinant of secretory organelle size in endothelial cells.
Sec22b silencing results in unlinked Golgi ribbon
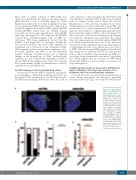
Since the size of nascent WPB is regulated by incorpora- tion of multiple so-called VWF quanta from the TGN,24 we investigated TGN morphology in Sec22b-depleted cells. TGN46 immunostaining showed that while the TGN in
control cells had a compact morphology, shSec22b-treated cells exhibited a dispersed TGN morphology, consistent with an unlinked Golgi ribbon (Figure 2A and B). Quantification of the area that encompassed the entire TGN46 immunoreactivity in shSec22b and shCTRL cells revealed that, due to their fragmentation, TGN in Sec22b- depleted cells extended to a significantly larger intracellu- lar area than the compact TGN in control cells (Figure 2C). The crucial role for Sec22b in maintaining Golgi integrity is not limited to endothelial cells, as illustrated by a similar effect on Golgi morphology in Sec22b-depleted HEK293T cells (Online Supplementary Figure S4). It has previously been described that unlinking Golgi stacks using depletion of Golgi matrix proteins or nocodazole gives rise to short- er WPB.24 When evaluating WPB length in shSec22b cells with compact versus dispersed TGN we also observed that in those cells in which the Golgi was dispersed, WPB were on average shorter than in those with intact Golgi (Figure 2D), which suggests that the reduction in WPB length after Sec22b depletion is (at least partly) a consequence of Golgi disintegration.
Sec22b silencing results in decreased von Willebrand factor trafficking to the Golgi and retention of von Willebrand factor in the endoplasmic reticulum
As Sec22b has been associated with membrane fusion events during anterograde and retrograde trafficking between the ER and Golgi,31 we evaluated VWF traffick-
A
Figure 2. Sec22b depletion results in trans-Golgi net- work fragmentation. (A) Immunofluorescent stain- ing of trans-Golgi network (TGN46) in control and Sec22b-depleted cells (blue channel: Hoechst nuclear staining). (B) Quantification of TGN dispersal in control and Sec22b knockdown (KD) endothelial cells (EC). (C) Quantification of TGN area coverage in shCTRL and shSec22b EC (n=5, t- test with Welch correction, ****P<0.001). (D) Weibel- Palade body (WPB) length in Sec22b KD EC with com- pact versus dispersed TGN (n=3, t-test with Welch cor- rection, ****P<0.0001).
B
CD
haematologica | 2021; 106(4)
1141