Page 131 - 2021_03-Haematologica-web
P. 131
HLA-G corrects dysfunction of immune cells in ITP
AB
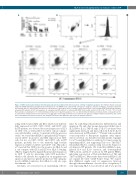
Figure 4. rhHLA-G attenuated immune thrombocytopenia patient peripheral blood mononuclear cell-induced platelet apoptosis. Recombinant human leukocyte antigen-G (rhHLA-G)-modulated peripheral blood mononuclear cells (PBMC) were cultured with autologous or allogeneic platelets for 4 hours, and platelet apoptosis was assayed with JC-1 mitochondrial potential test. (A) Apoptosis of autologous (Auto) or healthy control (Ctr) platelets cocultured with PBMC from immune thrombo- cytopenia (ITP) patients (n=17); and apoptosis of autologous and ITP platelets co-cultured with PBMC from healthy controls (n=15; n=8). *P<0.05; **P<0.01. (B) Representative scattergrams of JC-1 mitochondrial potential test for platelet apoptosis. JC-1 is a mitochondrial membrane potential-sensitive carbocyanine probe. JC-1 monomers emit green fluorescence whereas JC-1 aggregates emit orange-red fluorescence. Platelets were gated according to forward scatter (FSC), side scatter (SSC), and CD41a, then platelet apoptosis was analyzed. The dots in the right lower gate represent apoptotic platelets.
acting with receptors ILT2 and ILT4, which were differen- tially expressed on CD4+, CD8+, CD14+, and CD19+ cells. In ITP patients, we observed decreased expression of ILT2 on CD4+ cells, as well as ILT4 on CD14+ cells in compari- sion with healthy controls. Consistent with the previous study,32 we found that rhHLA-G upregulated ILT4 expres- sion in CD14+ monocytes, and ILT2 expression in CD4+ cells, but not on CD8+, CD14+, and CD19+ cells in ITP patients, probably due to the relatively high baseline expression of ILT2 on CD14+ and CD19+ cells. Shiroishi et al.36 reported that CD8 is also the receptor for HLA-G, and it can compete with ILT2 and ILT4 for HLA-G binding. sHLA-G triggers Fas/Fas-Ligand (FasL)-mediated apoptosis in CD8+ T cells by interacting with CD8 molecules,37 which could alleviate the suppression on megakaryocyte apoptosis and increase platelet production.38 ILT upregula- tion by rhHLA-G indicates that sHLA-G could also regu- late immune cells.
Cytokines are crucial factors in maintaining self-tole-
rance by controlling cell proliferation, differentiation, and migration. We and other groups demonstrated that type 1 (IL-2, IFN-a, IL-12, TNF-a), type 17 (IL-17) cytokines were significantly increased and type 2 (IL-4, IL-5, IL-10, IL-13) were decreased in ITP patients.39-41 Patients whose platelet count responded to therapeutic regimens often experi- enced correction of a disturbed cytokine profile. Agaugué S et al.42 found that HLA-G contributed to tumor escape by expanding MDSC and supporting Th2 versus Th1/Th17. Meanwhile, HLA-G could also help restore the balance of cytokines in ITP patients. We found that rhHLA-G upre- gulated IL-4 and IL-10 secretion, and downregulated TNF-a, IL-12, and IL-17 secretion by ITP patient PBMC, indicating a promotion of Th2 and inhibition of Th1 and Th17 by rhHLA-G. Our results demostrated rhHLA-G did not expand the CD4+ CD25+ Foxp3+ Tregs. We suspect that rhHLA-G might exert its suppressive function through other mechanisms. A novel subset of regulatory T cells expressing HLA-G was described, and it was proven
haematologica | 2021; 106(3)
777