Page 133 - 2021_03-Haematologica-web
P. 133
HLA-G corrects dysfunction of immune cells in ITP
MDSC population, which may inhibit CD4+ T-cell prolif- eration and CTL-mediated platelet lysis.46 Pickup et al.47 found that G-CSF promoted differentiation of CD11b+ Ly6G+cells, increased IL-10 secretion from myeloid cells and inhibited T-cell proliferation by upregulating inducible nitric oxide synthase, arginase, IL-6, IL-10, IL- 1β, and vascular endothelial growth factor, which are beneficial and protective in autoimmune diseases. On the other hand, IL-10 and IL-1β combined with GM-CSF and/or IL-2 are able to induce HLA-G protein expression.48 This may initiate a possible positive feed- back loop connecting cytokines and immune cells, and supporting immune tolerance.
Binding of HLA-G to its cognate receptor ILT may repair the disturbed cytokine profile and restore the immunosuppressive functions of various cells, such as T cells and DC. In our study, rhHLA-G could not affect platelet apoptosis directly, while rhHLA-G modulation attenuated platelets apoptosis when cocultured with PBMC from ITP patients, indicating the protective effect of HLA-G is through modulation of PBMC. sHLA-G interacts with CD8 molecules and induces apoptosis of cytotoxic T cells.37 In addition, HLA-G can suppress the cytolytic function of CD8+ T cells through downregula-
tion of granzyme B expression, and impair perforin gran- ules polarization toward target cells,49,50 which might explain its protective effect to platelets. Therefore, rhHLA-G may be a potential strategy to protect their own platelets from destruction in clinical practice.
By reprogramming the cytokine profile in ITP patients, HLA-G established an optimal environment for the impaired cell populations, such as DC, to regain their tolerogenic function. We found that lower expression of mHLA-G and ILT4 on CD14+ cells from ITP patients, con- firming the loss of immune tolerance in ITP. However, the effect of HLA-G on DC is still controversial. Liang et al.51 demonstrated that HLA-G-treated DC maintained a tolerogenic phenotype CD80low CD86low HLA-DRlow, and HLA-G inhibited DC maturation via IL-6 signaling path- way. However, Le Friec et al.52 found that sHLA-G inhibits human DC mediated T-cell proliferation without altering DC maturation processes. In order to determine the func- tion of DC in ITP patients as well as the protective role of HLA-G, we sorted CD14+ cells and isolated DC in vitro, and then modulated them with rhHLA-G. As expected, binding of HLA-G to ILT2/4 on DC suppressed the expres- sion of costimulatory molecules and showed decreased ability to stimulate T-cell proliferation. These results indi-
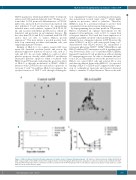
A
BC
Figure 6. HLA-G treatment alleviated thrombocytopenia in a murine model of immune thrombocytopenia. (A) Capture of human leukocyte antigen-G (HLA-G) by AuNP compared with control AuNP is shown in the image from the electron micrograph. (B) Survival rate and platelet counts in a murine model of immune thrombo- cytopenia (ITP) from group 1-4 (n=7). (C) Platelet counts in a murine model of ITP from group 1-4 (n=7). The data are expressed as platelet counts (1x109/L) ± stan- dard error of the mean over time (days).
haematologica | 2021; 106(3)
779