Page 92 - 2021_02-Haematologica-web
P. 92
C. Lagresle-Peyrou et al.
ABC
DE
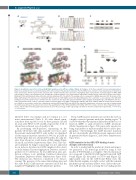
Figure 1. Identification and effects of the p.G12R RAC2 mutation on the GTPase activity of RAC2. (A) Pedigrees of the three patients from two unrelated kindred. Black boxes and black circles respectively represent the affected male (P1) and affected females (P2, P3). White boxes and circles respectively represent unaffected males and females. Arrows represent the probands and a double horizontal bar represents consanguinity. (B) A representative electropherogram of RAC2 DNA sequencing for control cells and patient cells, showing the c.34G>A mutation. (C) A representative immunoblot of RAC2 protein expression in lysates from control fibroblasts (Ctrl) and fibroblasts derived from the affected individuals (P1, P2, and P3). The loading control corresponds to GAPDH expression. (D) 3D models of the RAC2 G12R mutant. The structures are shown with GDP (left panel) or GTP (right panel) in the G1 binding pocket. For each model, a close-up view of the GDP/GTP binding pocket (dotted brown circle) is shown below the overall view. The figure was generated with the pymol program (www.pymol.org/). (E) HEK293T cells were either not transduced (NT, control) or transduced with a lentiviral empty vector (WPI) containing the wild-type (WT) form of RAC2 cDNA, the mutated form described here (G12R) or (as a positive control) the constitutively activated GTP-bound RAC2 form (G12V). Two days after transduction, cells were recovered for analysis using the G-LISA assay (15 μg of total protein per well) for the quantification of the GTP-bound RAC2 form (RAC2 GTP). The results come from three independent experi- ments, and the table below the graph represents the mean of the percentage of GFP expressing cells (GFP+) in the three independent experiments. ***P<0.001; ****P<0.0001.
inherited defect was intrinsic and not extrinsic (i.e., not micro-environmental) (Table 1). No other clinical symp- toms have been reported so far for these patients (12 and 21 years after HSCT for P1 and P2, respectively), both of whom have achieved a good quality of life.
By performing whole-genome sequencing on the patients’ fibroblasts (the only available cell source, since the patients underwent HSCT early in life), we identified a heterozygous missense mutation (c.34G>A, p.G12R) in the RAC2 gene that was absent in P3’s father, the only rel- ative from whom we could obtain a DNA sample (Figure 1A and B, and Online Supplementary Figure S1A). This vari- ant (confirmed by Sanger sequencing) was not annotated in our in-house database (n=14,154 samples in the cohort) or in the human Genome Aggregation Database, and was predicted to be deleterious by four different in silico pre- diction software tools, including Combined Annotation Dependent Depletion (Online Supplementary Figure S1B). The mutation was, therefore, considered to be disease- causing. The presence of the same clinical phenotype and the same RAC2 G12R missense mutation in P2 and her daughter P3 highlighted an AD inheritance pattern.
The p.G12R missense mutation is located in the G1 box, a highly conserved guanine nucleotide binding region.14 It is noteworthy that this mutation differed from the loss-of- function (LOF) or gain-of-function (GOF) mutations (Online Supplementary Figure S1A) previously reported as being responsible for mild neutrophil defects and/or lym- phopenia.15-20 Interestingly, the G12R missense mutation did not substantially alter RAC2 protein expression level in patient fibroblasts (Figure 1C and Online Supplementary Figure S1C).
G12R mutation in the GDP/GTP-binding domain disrupts cell homeostasis
In order to understand the mutation’s functional impact, we generated a 3D homology model by using in silico models of WT RAC221 as a template. The structural mod- els correspond to the RAC2 G12R mutant form with GDP bound (Figure 1D, left panel) or GTP bound (Figure 1D, right panel) state. In the GTP-bound RAC2 state, the bulky flexible arginine is in tight contact with the terminal phosphate group and may induce steric clashes that pre- vent GAP proteins from accessing the G1 binding pocket
406
haematologica | 2021; 106(2)