Page 215 - 2021_02-Haematologica-web
P. 215
PolyP enhances uPA-mediated plasminogen activation
AB
C
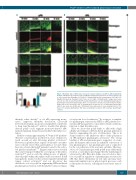
Figure 7. Real-time lysis of fibrin clots reveals the cofactor function of polyP on uPA-mediated fib- rinolysis. Fibrinolysis by exogenous PA (75 nM) was monitored by fluorescent confocal microscopy of clots formed from fibrinogen (2.65 mM, 9% DyLight 488-labeled), plasminogen (1.25 mM, 20%
(5 mM) ± 328 mM polyP
every 15 seconds (s). (A) uPA and (B) tPA mediated fibrinolysis over time showing fibrinogen (green), plasminogen (red) and merged images where co-localisation is visualised as yellow. Representative image from n=3, scale bar=10 mm. (C) Quantification of lysis time in s as determined by the time taken to lyse scan area (134.8 mm x 134.8 mm). Data expressed as mean ±standard error of the mean, n=3. polyA: polyphosphate; tPA: tissue plasminogen activator; uPA: urokinase plasminogen
DyLight 633-labelled), thrombin (0.25 U/mL), CaCl activator.
2
65
. Images were taken
thrombi, either directly38 or via uPA expressing mono- cytes,39 improves thrombus dissolution. Cross-talk between neutrophils, monocytes and platelets contributes to deep vein thrombosis40 and it is feasible that platelet- derived polyP could augment monocyte-derived uPA- mediated plasmin formation and facilitate thrombus reso- lution.
Platelets contain approximately 0.74 nmol/108 platelets1 therefore concentrations in whole blood are estimated to reach around ≈3 mM following platelet activation.25 However, in platelet dense regions of thrombi concentra- tions could far exceed this; particularly as we and others have also shown that polyP remains bound to the activa- ted platelet membrane.26,41 Elegant studies have revealed that solute transport within the core regions of thrombi is restricted42-44 and as such the diffusion rate of platelet- derived polyP within this milieu will be restricted. PolyP nanoparticles formed on the activated platelet membrane trigger contact activation26 and our laboratory has described a role for polyP in augmenting FXIIa-mediated plasminogen activation, with the platelet surface acting as
a focal point for colocalization.14 By acting as a template for plasminogen activation by FXIIa or uPA, platelet-asso- ciated polyP nanoparticles could, under certain conditions, function to limit thrombus size.
Here we show that polyP binds to uPA with a high affinity and enhances uPA-mediated plasmin generation thereby augmenting the rate of fibrinolysis. This is in sharp contrast to the inhibitory effect of this polyanion on tPA-mediated plasminogen activation.4 The strong binding of uPA and plasminogen is suggestive of a tem- plate mechanism that results in enhanced conversion of Glu-plasminogen to Lys-plasminogen and is supported by the colocalization of the reactants on fibrin. The inter- action of Glu-plasminogen with polyP may additionally result in a conformational change thereby facilitating its activation by uPA. Our data define a novel cofactor func- tion of polyP in regulation of plasminogen activation by uPA that drives fibrinolysis in real time and may have important implications for physiological processes such as thrombus resolution, cell migration and tissue remod- eling.
haematologica | 2021; 106(2)
529