Page 214 - 2021_02-Haematologica-web
P. 214
C.S. Whyte and N.J. Mutch
AB
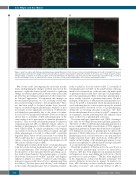
Figure 6. polyP colocalizes with fibrinogen and plasminogen during fibrinolysis. Fibrin clots were formed containing fibrinogen (2.65 mM, 9% DyLight 550-labeled),
(5 mM). (A) polyP and plas- minogen largely accumulate in the knotted regions of fibrin. (B) Fibrinolysis was initiated by exogenous uPA (75 nM). Plasminogen and polyP strongly colocalize at
Glu-plasminogen (1.25 mM, 20% DyLight 633-labeled) 328 mM Cascade-blue labeled polyP70 (CB-polyP), thrombin (0.25 U/mL), CaC
the lysis front during clot lysis, as indicated by the arrows. Scale bars =10 mm. polyP: polyphosphate; uPA; urokinase plasminogen activator.
Our recent study19 investigating the molecular mecha- nisms underpinning the changes in fibrin structure in the presence of platelet-derived polyP revealed a significant change in fibrin polymerisation which stunts protofibril growth; thus providing an explanation for the characteris- tic ‘knotted’ appearance.4 Downstream this impacts on the mechanical properties of a clot, reducing overall stiff- ness and increasing its ability to deform plastically.19 Here, we find that polyP is localized within these ‘knotted’ regions of fibrin alongside plasminogen. Analysis of lysis in real-time reveals significant acceleration of uPA-media- ted fibrinolysis presumably due to the direct colocaliza- tion of cofactor, enzyme and substrate. Previous work has shown that co-assembly of uPA and plasminogen on the same surface is not a prerequisite to stimulate plasmin for- mation.27 This crosstalk mechanism permits localization of plasminogen and uPA on different cellular surfaces or binding of plasminogen to fibrin while uPA is associated with cellular uPAR. A similar mechanism could explain our current observations; that is uPA is localized on polyP while plasminogen is bound to either polyP or partially degraded fibrin. Future work is necessary to ascertain the effect polyP may have on plasminogen activation on the surface of monocytes and neutrophils, which express both uPA and its cellular receptor uPAR.
Plasminogen circulates in the native or Glu-plasminogen form but can be cleaved by plasmin at Lys77-Lys78 to gener- ate Lys-plasminogen. This cleavage prompts changes in the properties of the zymogen thereby providing a posi- tive feedback mechanism.28 Lys-plasminogen is a conside- rably better substrate for both tPA29 and uPA30 and displays enhanced affinity for fibrin.31,32 Surface-bound Glu-plas- minogen is more readily cleaved to Lys- plasminogen than in solution.33 We have previously shown,14 and confirmed in this study, that plasminogen, but not plasmin, bindsdi-
l2
rectly to polyP, as does the activator uPA. Co-assembly of Glu-plasminogen and uPA on the polyP surface will aug- ment local concentrations of the reactants. An initial spark of plasmin formation will drive cleavage of Glu-plasmino- gen to Lys-plasminogen. Once formed Lys-plasminogen is more readily activated to plasmin than native Glu-plas- minogen. Stimulation of uPA-mediated plasminogen acti- vation by polyP is diminished when Lys-plasminogen is used, indicating that its cofactor function may lie in initial surface-mediated conversion of Glu- to Lys-plasminogen, ultimately accelerating plasmin formation. We have shown that plasmin does not bind to polyP4 or impact on its enzymatic activity indicating that the enhanced lysis arises at the level of plasminogen activation.
The lysine analogue, tranexamic acid (TXA), downregu- lates lysis by blocking tPA-mediated plasmin generation. The effect of TXA on uPA-mediated plasmin generation is more complex, with high concentrations of TXA augment- ing plasmin generation, despite this fibrinolysis is still inhib- ited. Enhanced activation of plasminogen by uPA in the presence of TXA is indicative of a conformational change in plasminogen, from a closed structure to an open and more readily activated form.34 Our observations with polyP are similar, in that binding of Glu-plasminogen appears to aug- ment its susceptibility to uPA-mediated cleavage indicative of a conformational change in the protein.
The fibrin specificity of tPA has led to the view that it is the dominant plasminogen activator in hemostasis, whereas, uPA has been implicated in plasmin-mediated cell migration, tissue remodelling and activation of latent growth factors and cytokines.35 TPA has been implicated in the degradation of deep vein thrombi in humans36 but genetic deficiency in mice has no impact. In marked con- trast, uPA deficiency in mice markedly impairs venous thrombus resolution37 and conversely delivery of uPA to
528
haematologica | 2021; 106(2)