Page 142 - 2021_02-Haematologica-web
P. 142
P.J. Hohensinner et al.
Statistics
Sample groups were compared using a paired Student t-test using SPSS 21 (IBM, CA, USA). P-values ≤0.05 were considered statistically significant. The number of individual donors per experiment is given in the figure legends. All graphs depict the mean values ± standard deviation.
Results
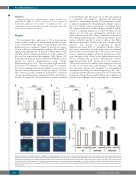
We determined the expression of TF in macrophages under baseline condition as well as under polarized condi- tions to understand the impact of macrophages and their polarization on coagulation. When TF protein was meas- ured in cell lysates of human macrophages, IL-4 and IL-13 significantly upregulated TF protein content whereas LPS and IFN-γ did not change protein levels of TF compared to the levels in unpolarized macrophages (M0) (Figure 1A). Polarization conditions did not influence viability as deter- mined by lactate dehydrogenase assay (Online Supplementary Figure S1A). TF is associated with extracellu- lar vesicles. We, therefore, determined the TF concentra- tion in macrophage-derived extracellular vesicles at base- line and under polarization conditions. Again, TF levels were increased under alternative polarization conditions whereas proinflammatory stimulation did not alter TF pro- tein levels compared to the levels in microvesicles obtained
from M0 (Figure 1B). The main role of TF is the initiation of coagulation. We, therefore, analyzed the functional capacity of macrophage-derived TF-bearing microvesicles to initiate coagulation by determining the change of factor X to its activated form via generation of activated factor VII. Again, polarization of macrophages with IL-4 and IL- 13 led to a marked induction of active TF microvesicles (Figure 1C). To rule out contamination and hence low- grade induction with LPS during alternative polarization we determined the induction of TF under alternative polar- ization with IL-4 and IL-13 with or without the TLR-4 inhibitor polymyxin B. Similar results were obtained in the presence and absence of polymyxin B (Online Supplementary Figure S1B). To determine whether TF pro- tein can be detected at an early time point, we analyzed microvesicles secreted after 6 h for TF content. We did not observe significant changes with any polarization condi- tion, although we did observe a non-significant increase for TF in alternatively activated macrophages (Online Supplementary Figure S1C). Besides its role in coagulation, TF has been reported to influence the migratory behavior of cells via its interaction with integrins. This cross-talk was described, among other examples, for cell migration on laminin 5 resulting in reduced migration of TF+ cells.22 To analyze whether increased expression of TF reduces the formation of filopodia we analyzed filopodia formation of polarized macrophages when migrating into a laminin 5-
ABC
D
Figure 1. Tissue factor production after polarization of macrophages. (A) Tissue factor (TF) protein was determined on extracellular vesicles from supernatant (n=6) and (B) from lysed cells (n=7) using a specific enzyme-linked immunosorbent assay as indicated in the Methods section. (C) TF activity on extracellular vesicles from polarized macrophages was evaluated using an activity assay as indicated in the Methods section (n=13). Values are given in pg/mL and represent mean values ± standard deviation. (D) Capability of human polarized macrophages to form filopodia when migrating onto laminin-coated areas was evaluated by cytoskeletal stain- ing (n=3). M0: unpolarized macrophages; M(LPS+IFN): classically activated polarized macrophages; M(IL-4+IL13): alternatively activated polarized macrophages; ns: not significant.
456
haematologica | 2021; 106(2)