Page 106 - 2021_02-Haematologica-web
P. 106
M. Hu et al.
lates the cell cycle and apoptosis, most likely depending on the cellular contexts. It was well recognized that the loss of HSC quiescence can bring about a transient aug- mentation of phenotypic HSC but eventually compromis- es their function.6 These results could explain our observa- tion that miR-21D/D HSC have a diminished long-term reconstituting capacity. Notably, the reciprocal transplan- tation assay validated that the defects manifested in miR- 21-deficient HSC are cell-intrinsic. In addition, we observed a myeloid bias in recipients transplanted with miR-21D/D BM cells, which is consistent with the changes in non-transplanted miR-21D/D mice.
In an effort to characterize the molecular mechanisms by which miR-21 regulates HSC homeostasis, we per- formed a microarray analysis. Notably, we observed a marked downregulation of the NF-κB pathway when
miR-21 was deleted. It is well established that the NF-κB transcription factor family plays a key role in various physiological processes, including cell proliferation, apop- tosis, inflammation and immune responses.41 Current studies using mouse genetic models have indicated that, although aberrant activation of NF-κB is not beneficial for hematopoiesis, basal NF-κB signaling is indispensable for HSC homeostasis.42 Interestingly, miR-21D/D HSC showed similar phenotypes to those of p65-null HSC.30 However, it is unknown whether miR-21 regulates HSC homeosta- sis and function via the NF-κB pathway. Further investiga- tions revealed that a previously recognized target of miR- 21, the tumor suppressor PDCD4,33,43 was obviously upregulated in HSC with miR-21 deficiency. However, there is controversy about the function of PDCD4 in reg- ulating NF-κB activity. Most studies have shown that
ABC
DEF
GHI
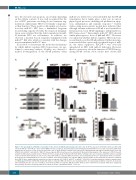
Figure 6. The upregulation of PDCD4 is responsible for the defects in miR-21-null hematopoietic stem cells. (A, B) The expression of PDCD4 in Lin-Sca1+c-Kit+ (LSK) cells from miR-21fl/fl and miR-21∆/∆ bone marrow (BM) (n=5 mice per group), determined by western blotting (A) and immunofluorescence (B), respectively. DAPI stain- ing indicates the nucleus of cells. Scale bar represents 5 mm. (C) Flow cytometric analysis of the expression of PDCD4 in LSK and long-term hematopoietic stem cells (LT-HSC) from miR-21fl/fl and miR-21∆/∆ BM (n=5 mice per group). MFI: mean fluorescence intensity. (D) Western blotting analysis of the expression of PDCD4, p-p65 and p65 in LSK transduced with control or PDCD4 (n=5 mice per group). (E, F) Normal LSK from CD45.2+ wild-type mice were transduced with the lentivirus carrying control or PDCD4, then transduced cells (6×103), mixed with CD45.1+ competitor BM cells (5×105), were transplantated into 10.0 Gy-irradiated CD45.1+ recipients. At 12 weeks after transplantation, the cell cycle of CD45.2+ donor-derived LT-HSCs in recipients’ BM (E), and the CD45.2+ donor chimerism in recipients’ peripheral blood (PB) (F) were analyzed by flow cytometry (n=6 mice per group). (G-I) CD45.2+ miR-21fl/fl or miR-21∆/∆ LSK (6×103) transduced with the lentivirus carrying control or shRNA against PDCD4 (sh-PDCD4), mixed with CD45.1+ competitor BM cells (5×105), were transplanted into 10.0 Gy-irradiated CD45.1+ recipients. At 12 weeks after transplantation, the cell cycle of CD45.2+ donor-derived LT-HSC in recipients’ BM (H), and the CD45.2+ donor chimerism in recipients’ PB (I) were analyzed by flow cytometry (n=6 mice per group). All data are shown as means ± standard deviation. **P<0.01.
420
haematologica | 2021; 106(2)