Page 56 - Haematologica-5
P. 56
788
A. Khaibullina et al.
microangiopathy, focal segmental glomerulosclerosis (FSGS), membranoproliferative glomerulonephritis, and albuminuria.5-8 Two major disease mechanisms of chronic kidney disease in SCD have been proposed: 1) hemolysis- endothelial dysfunction leading to vasculopathy; and 2) inflammation and hyper-viscosity leading to vaso-occlu- sion.9 Intrarenal RBC hemolysis was suggested to be a trig- ger for both mechanisms.
Spleen, the physiological site of RBC removal from the circulation, is abnormal in SCD patients. The functional asplenia is likely to increase the rates of intravascular hemolysis. Sickling of RBCs and intra-organ hemolysis stimulate infiltration by circulating monocytes and their dif- ferentiation into macrophages. Endocytosis of RBC lysate products affects macrophage phenotypes.10,11 Intravascular RBC hemolysis also releases lysate products that impair endothelial function leading to chronic vasculopathy. Vascular endothelium and monocytes are activated in SCD patients, and monocyte numbers are increased.12-14 Activated macrophages express matriptase-1 (MT-SP1) which is one of the proteases that cleavages and activates circulating macrophage stimulating protein 1 (MSP1).15,16
MSP1 was shown to accumulate in glomeruli in the rat model of anti-Thy1 glomerular disease; its neutralization by antibodies reduced serum creatinine and proteinuria, and protected rats from glomerular injury.17 MSP1 is a plas- ma protein secreted by liver and circulated as a single-
chain, biologically inactive pro-MSP1. It is activated by proteolytic cleavage of Arg483-Val484 bond by either serum proteases or proteases expressed on the cell surface.18 Pro-MSP1 diffuses into local tissues where it is activated by proteolytic cleavage and plays a role in the tissue injury or repair.18 Activated MSP1 binds to and acti- vates a cell surface receptor tyrosine kinase, Recepteur d'Origine Nantais (RON).19 We hypothesize that endocy- tosis of RBC lysis products by kidney-infiltrating macrophages stimulates expression of MT-SP1, which then locally activates circulating MSP1. We further hypothesize that MSP1 binds to RON tyrosine kinase receptor and activates glomerular endothelium in SCD. We test these hypotheses using a humanized mouse model of SCD (Townes) which recapitulates several hematologic manifestations of human SCD, including renal vascular occlusion, as well as vascular, tubular and glomerular changes.20,21 Here we showed that glomerular disease in SCD mice was associated with endothelial injury, increase in renal macrophage infiltration, and glomerular MSP1 accumulation. In vitro, treatment of cul- tured human macrophages with hemin, a breakdown product of hemoglobin, or RBC lysate significantly increased expression of MT-SP1. In cultured human renal glomerular endothelial cells, MSP1 treatment induced phosphorylation of RON downstream signaling ERK and AKT kinases, increased expression of von Willebrand fac- tor (vWF) and cell motility, and induced re-organization of
A
B
C
D
E
F
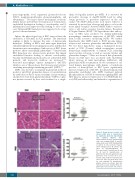
Figure 1. Renal disease in sickle cell disease (SCD) mice is characterized by significant glomeruli hypertrophy
P=4x10-17
P=5x10
Representative pictures of hematoxylin and eosin staining (H&E) (A and B), and periodic acid–Schiff staining (PAS) (C and D) of renal sections. Squares show enlarged areas (B and D). Bar sizes on microphotographs are 100 μm (A and C) and 40 μm. (B and D). (E and F) Quantification of glomeruli size (E) and capillary size per glomeruli cross section (F) is per- formed using CellSens Standard soft- ware. Five mice per group were used for each staining. For quantification graphs, means are shown. Each dot represents a value obtained from one glomerulus cross-section. Ctrl: control.
-9 and capillary dilation. (A-D)
haematologica | 2018; 103(5)