Page 105 - Haematologica-5
P. 105
MSH6 haploinsufficiency contributes to resistance
mide,28,36,37 which produces DNA O6-methyguanine, a lesion structurally similar to 6-TG.38 Our data demonstrat- ing decreased sensitivity of MSH6 knockdown cells to temozolomide support the hypothesis that MMR defi- cient clones gain an advantage under this selective pres- sure leading to resistant recurrences.27,39 The difference in TMZ sensitivity between the two UOCB1 shRNA knock- down cell lines could be due to the interplay between MSH6 and SETD2 protein levels since UOCB1 cells have a copy number loss of SETD2 (NA Evensen et al., 2018, unpublished data) and there is a greater reduction of MSH6 with shRNA1 compared to shRNA2. SETD2, the gene that codes for the methyltransferase responsible for the trimethylation of H3K36 that serves as the docking site for MSH6,40 is among the epigenetic regulators commonly found mutated in relapse patients.41 Ongoing studies in our lab are focused on identifying the relationship of epigenet- ic readers, writers, and erasers, such as SETD2, MSH6, and WHSC1 in chemoresistance.
Mechanistically, our in vitro and in vivo data support the hypothesis that the delayed cytotoxic response to thiop- urines is due to the MMR system recognizing a mismatch and initiating futile, damaging DNA repair that ultimately
leads to apoptosis.20,42,43 This pathway is not fully activated in cells with reduced MSH6 because the mismatch goes undetected, allowing these cells to tolerate excess TGN mismatches and, ultimately, to continue to survive and proliferate while under treatment. Our data provide evi- dence that, upon recognition of mismatches, NT cells slow their progression through S phase by activating Chk1 as they begin to repair their DNA. Due to the mis- match being on the daughter strand, the excision/repair process is unsuccessful, and over time nicks build up in the DNA, demonstrated by increased levels of γH2AX. Eventually, the damage becomes overwhelming and cells initiate apoptosis, as shown by increased p53. MSH6 shRNA1 cells exhibited minimal to no change in cell cycle, activation of Chk1, or increased γH2AX and p53. The moderate changes observed with the MSH6 shRNA2 cells highlight the idea that even a more modest reduction in MSH6 expression could lead to subtle changes that have a significant impact on chemoresistance. The MMR defi- cient Reh cells also had no alteration in their cell cycle, suggesting that recognition of mismatches by MutSa is not sufficient for full activation of this cascade, but rather damage induced by the repair, which is orchestrated by
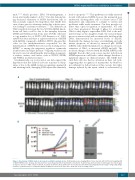
A
BC
Figure 6. Knockdown of MSH6 leads to decreased sensitivity to purixan in vivo. (A) Bioluminescence imaging (BLI) of mice injected with UOCB1 non-targeting (NT) or MSH6 shRNA1 cells. Six days after injection mice were imaged and then randomized to treatment. Treatment was started on day 7 and images were taken again on days 13 and 17. C: PBS control treatment; T: purixan treatment. (B) Quantification of total flux was determined by analyzing the BLI images using Living Image software. (C) Western blot to confirm knockdown of MSH6 in cells used to inject mice. Actin was used as loading control.
haematologica | 2018; 103(5)
837