Page 37 - Haematologica-April 2018
P. 37
Erythropoietin receptor mutations in PFCP
EPOR p.Gln434Profs*11 (EPOR FS) share the same five- amino acid terminal sequence (MDTVP). MTT-like assays showed that erythropoietin hypersensitivity was induced by EPOR p.Pro438Metfs*6, but not by EPOR p.Pro443* (Figure 5B), similarly to the results obtained with EPOR p.Gln434Profs*11 (EPOR FS) and EPOR p.Gln444* (EPOR STOP), respectively. We also studied two proximal non- sense mutations, EPOR c.1195G>T (p.Glu399*)11 and c.1273G>T (p.Glu425*)10 (Figure 5A), which are responsi- ble for more extensive truncations (109 and 83 amino acids, respectively) and the loss of seven of the eight cyto- plasmic tyrosine residues, retaining only Tyr-368 (Table 1, Figure 6A,B). Interestingly, these nonsense mutants, unlike EPOR p.Pro434* and p.Gln444*, were able to confer ery- thropoietin hypersensitivity to Ba/F3 cells (Figure 5C). These results show that extensive truncations, unlike shorter ones, are sufficient per se to induce the erythropoi- etin hypersensitivity phenotype. In comparison, erythro- poietin hypersensitivity induced by frameshift EPOR
AB
mutations is due to a distinct common mechanism based on the appearance of new amino acid sequences.
Discussion
In this study, we identified a new germline heterozygous EPOR mutation, c.1300dup (p.Gln434Profs*11) in a patient suffering from PFCP. Like the other mutations already described in this pathology, c.1300dup is located in exon 8 of EPOR. The frameshift generates a new cytoplasmic tail of ten amino acids and a premature stop codon at position 444 leading to the loss of 64 amino acids in the C-terminal part of the receptor. Two other mutations, c.1281dup (p.Ile428Tyrfs*17)7 and c.1288dup (p.Asp430Glyfs*15),4 leading to a similar truncation at position 444 had been previously reported but, as for most other EPOR mutants, no extensive functional study had been carried out. Here we show that EPOR p.Gln434Profs*11 is a strong gain-of-
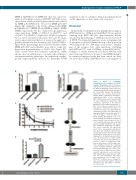
Figure 3. Effects of c.1300dup (p.Gln434Profs*11) mutation on EPOR stability, dimerization and cell surface expression. (A) Cell-surface expression of the different EPOR was assessed by flow cytometry using PE fluorescent labeling of the extracellular HA-tag. The histogram shows the ratio of mean fluorescence intensiy (MFI) of PE-labeled cell-surface erythropoietin (EPO) on the respective MFI of GFP. Results are the mean ± SEM of seven independent experiments. (B) Cell-surface expression of the different EPOR was assessed with radiolabeled 125I- EPO. The results are expressed in cpm normalized to EPOR WT. The number of cell-surface receptors was determined by comparison between the radioactivity of transduced Ba/F3 cells and parental UT-7 cells that express 7000 receptors. (C) EPOR stability. Ba/F3-EPOR cells were incubated with cycloheximide for different times (0 min, 15 min, 30 min, 1 h, 2 h, 4 h, 6 h) and HA expression was studied by western blotting. HA-EPOR and β-actin were quantified using Image J software. The curves represent the HA/β-actin ratios. Three independent experiments were done. (D) Schematic representation of split Gaussia princeps luciferase com- plementation assay used to test EPOR dimerization in HEK293-derived BOSC cells. (E) Dimerization of human EPOR monomers was assessed by split Gaussia luciferase assay in steady-state condi- tions, in the absence of EPO in HEK cells. Close proximity between the C-terminal cytosolic domains of EPOR is significantly promoted by EPOR FS. The results repre- sent the mean ± SEM from three inde- pendent experiments, each performed with eight biological replicates per condi- tion. For each experiment, raw values were normalized to the average of the EPOR WT condition before pooling the data of all experiments together. Unpaired two-tailed t-test with Welch correction, ****P<0.0001.
CD
E
haematologica | 2018; 103(4)
581