Page 119 - Haematologica-April 2018
P. 119
CD83 and Hodgkin lymphoma
(brentuximab).10 As CD83 is inducible, it is possible that either drug induced or inflammatory activation would induce greater CD83 expression on HRS cells. We also identified serum sCD83 as a potential disease marker. Its immunosuppressive effect was reversed by anti-CD83 mAb at levels readily obtained in vivo. We predict that 3C12C would target HL cells directly through ADCC, but it has the additional therapeutic effect of reversing the inherent immunosuppressive effect of CD83. Such a syn- ergistic response has the potential to have a significant clinical effect with limited toxicity.
Recent studies revealed the impact of tumor microenvi- ronment on tumor progression and therapy. HL is a lead- ing example. The low frequency malignant HRS cells secrete several factors and generate a surrounding infil- trate of immune cells that contribute to the pathogenesis of the disease.35-37 CD83 appears to be involved in this process. We previously demonstrated that the transfer of membrane proteins on myeloma cells to T cells disrupted the immune response and was associated with poor prog-
A
nosis.38 We found that HL tumor cells express CD83 and can transfer surface CD83 molecules by trogocytosis. CD83 transfer from KM-H2 to T cells in vitro was consis- tent with the finding that some lymphocytes in the lymph node biopsy samples, especially in CD83 high expression patients, expressed CD83. The proportion of Treg in the trogocytosed CD83+CD4+ T cells was not increased, but CD83+ T cells, especially CD4+ T cells, expressed a higher level of PD-1 than CD83– T cells. PD-1 and PD-1L interac- tion contributes to the immunosuppressive microenviron- ment of HL.39 Such PD-1high CD83+ T cells might become unresponsive in the tumor microenvironment.40 A CD83 target therapy might be combined with brentuximab and PD-1 blockage to enhance the clinical response.
The serum of some hematopoietic malignancies have increased levels of sCD83.20,30 The supernatant of HL cells inhibited T-cell proliferation, but this inhibitory effect was not related to Treg induction (data not shown). The anti- CD83 mAb, 3C12C, partially abolished the inhibition by KM-H2 supernatant. Thus, sCD83 from the supernatant
B
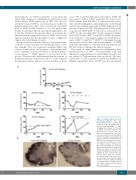
Figure 7. 3C12C reduced B cells in non-human primates. Five non- human primates were injected with 3C12C (1, 5, 10, 10 mg/kg, n=4) or human Immunoglobulin G (IgG; 10mg/kg, n=1) at days 0, 7, 14 and 21. Blood and serum samples were collected for cell counts (red cells, white cells and platelets), liver func- tion (ALP and AST levels) and kidney function (creatinine level) analysis. (A) CD19+ B cells were enumerated from PBMC of five animals by flow cytome- try. Dashed lines indicate the base cell number at day 0. *indicates one time point when WBC was extremely high on that animal. (B) A lymph node biopsy was taken at day 28 from 3C12C (10mg/kg) and control-treated animals. B cells stained with anti- human CD20 mAb on paraffin- embedded lymph node biopsy sam- ples are shown. One of the two similar results for the two animals receiving 10 mg/kg 3C12C showing reduced B-cell areas compared to the human IgG control animal.
haematologica | 2018; 103(4)
663