Page 193 - 2020_08-Haematologica-web
P. 193
Coagulation factor carboxylation in mammalian cells
AB
CD
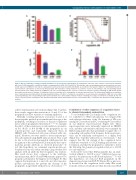
Figure 4. Effect of naturally occurring propeptide mutations on reporter-protein carboxylation. (A) Carboxylation efficiency of the chimeric reporter-protein with wild- type factor IX (FIX) propeptide or with its naturally occurring mutations N-9K, A-10T, and A-10V. The wild-type and corresponding mutant reporter-proteins were tran- siently expressed in HEK293 cells and the carboxylated reporter-protein was determined by ELISA, as described in the legend to Figure 1C. (B) Warfarin titration of reporter-protein carboxylation as directed by FIX propeptide with naturally occurring mutations at positions -9 and -10. The wild-type and corresponding mutant reporter-proteins were stably expressed in HEK293 cells. The corresponding reporter cells were cultured in complete medium containing 20 nM vitamin K with increasing concentrations of warfarin. (C) Inhibition efficiency of warfarin on the carboxylation of mutant reporter-proteins. The half-maximal inhibition concentration (IC50) of warfarin was determined from (B) using GraphPad software. (D) Effect of vitamin K concentration on reporter-protein carboxylation. HEK 293 cells stably expressing wild-type and corresponding mutant reporter-proteins were cultured with complete medium containing increasing concentrations of vitamin K. The effi- ciency of reporter-protein carboxylation was determined by ELISA and the half-maximal effective concentration (EC50) of vitamin K was determined using GraphPad software.
achieve half-maximal carboxylation (Figure 4D). Together, these results suggest that mutations at -9 and -10 of the propeptide are more sensitive to warfarin inhibition.
Naturally occurring mutations at position -4 and -1 of the propeptide preclude post-translational cleavage of the propeptide, resulting in secretion of the pro-coagulation factor with its propeptide still attached.29,30,34,35 To examine the effect of these mutations on carboxylation, we mutat- ed R-1 (R-1S) or R-4 (R-4Q) of the propeptide in our reporter-protein and transiently expressed them in HEK293 cells. Transfected cells were cultured with vita- min K. However, we were unable to detect reporter-pro- tein carboxylation using ELISA (data not shown). This could be due to the un-cleaved propeptide which prevents the recognition of the carboxylated Gla domain by the confor- mational specific antibody, as observed previously.29 To test this hypothesis, we examined reporter-protein car- boxylation from cell culture medium using western blot analysis with an antibody that recognizes Gla residues. Our result shows that, compared to the wild-type reporter-protein, the R-1S and R-4Q mutants appear to be properly carboxylated, but migrate slower (Figure 5). This suggests that mutations at -1 and -4 do not affect reporter- protein carboxylation but prevent the cleavage of the propeptide, in agreement with previous observations.29,30,34
Contribution of other sequences of coagulation factor to vitamin K-dependent carboxylation
To test whether the Gla domain of the coagulation fac- tor contributes to VKD carboxylation, we compared the carboxylation efficiency of the Gla domains of FIX (con- taining 12 Gla residues) and PC (containing 9 Gla residues) in our chimeric reporter-protein (Figure 6A). These reporter-proteins were transiently expressed in HEK293 cells, and their carboxylation efficiency was examined by ELISA using antibodies that specifically recognize the cor- responding carboxylated Gla domains. Both reporter-pro- teins can be efficiently carboxylated to a similar level (Figure 6C). Together with previous observations,7,13 this suggests that the Gla domain of the coagulation factor contributes very little to GGCX binding and substrate car- boxylation, and that the propeptide is sufficient to direct the following Gla domain of coagulation factors to GGCX for carboxylation.36
It should be noted that BGP propeptide has an unde- tectable affinity to GGCX in vitro,16 which has been sug- gested to be unnecessary for BGP carboxylation.14,15 To test this hypothesis, we removed BGP propeptide in the chimeric reporter-protein BGP-PC (Figure 6B), as previous- ly described in the coagulation factor study.7 Our result from the cell-based study showed that the removal of BGP
haematologica | 2020; 105(8)
2169