Page 191 - 2020_08-Haematologica-web
P. 191
Coagulation factor carboxylation in mammalian cells
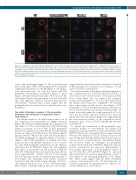
Figure 2. Localization of reporter-proteins by immunofluorescence confocal imaging. The reporter-protein factor IX gla-protein C (FIXgla-PC) with the propeptide of FIX or bone Gla protein (BGP) was transiently expressed in COS-7 cells. The transfected cells were cultured with complete medium containing 11 μM vitamin K. The carboxylated reporter-protein (carboxylated PC) was immuno-stained by the mouse anti-carboxylated FIXgla monoclonal antibody as the primary antibody, and Alexa Fluor-488 conjugated donkey anti-mouse IgG as the secondary antibody (green image). The total reporter-protein (PC) was probed by the sheep anti-PC polyclonal antibody as the primary antibody and the Alexa Fluor-568 conjugated donkey anti-sheep IgG as the secondary antibody (red image). The cell nucleus was stained by Hoechst 33342 (blue image).
cence confocal imaging (Figure 2). The reporter-protein (fused with either a FIX or BGP propeptide) was properly synthesized and directed to the ER (Figure 2, red images, total reporter-protein), but only the fusion with FIX propeptide was properly carboxylated (Figure 2, green image, carboxylated reporter-protein). The location of the carboxylated reporter-protein appears to be in both the ER and Golgi apparatus, which is consistent with previous observations.24 Together, these results suggest that the propeptide plays an essential role in coagulation factor car- boxylation.
The entire N-terminal sequence of the propeptide determines the efficiency of coagulation factor’s carboxylation
The alanine residue at -10 and leucine residue at -6 of the coagulation factor propeptide are highly conserved (Figure 1A). However, BGP propeptide has a glycine at -10 and valine at -6. The in vitro study shows that substituting alanine for glycine at -10 (G-10A) in BGP propeptide increased its affinity for GGCX 45-fold, and substituting leucine for valine at -6 (V-6L) increased its affinity approx- imately 100-fold.25 When both G-10A and V-6L are mutat- ed in BGP propeptide, its apparent affinity for GGCX is similar to that of FIX propeptide. To examine how these mutations affect carboxylation efficiency in vivo, we made the same BGP propeptide substitutions in the chimeric reporter-protein of FIXgla-PC (Figure 1C) and examined their effect on reporter-protein carboxylation in HEK293 cells. The V-6L mutant increased reporter-protein carboxy- lation approximately 20-fold and the G-10A mutant increased reporter-protein carboxylation approximately 7- fold (Figure 3A). Mutating both residues increased reporter-protein carboxylation approximately 23-fold, a carboxylation efficiency close to that of FIX propeptide. These results, consistent with our previous in vitro study,25
suggest that the conserved residues at positions -6 and -10 in the propeptide are essential for its binding to GGCX and for substrate carboxylation.
Factor X propeptide is the tightest binding propeptide of the coagulation factors, and carboxylation of FX has a slow turnover rate. Based on these observations and the above result (Figure 3A), we replaced the conserved residues of FX propeptide at -10 or -6 to that of the BGP in the chimeric reporter-protein of FIXgla-PC. We assumed these replacements would decrease the affinity of FX propeptide for GGCX and therefore increase the turnover rate of reporter-protein carboxylation. Unexpectedly, our results show that neither the single mutation (L-6V or A- 10G) nor the double mutation (L-6V/A-10G) increased reporter-protein carboxylation (Figure 3C), suggesting that the non-conserved residues of the coagulation factor’s propeptide play a role in GGCX’s binding and substrate carboxylation.
To explore the contribution of the propeptide’s non- conserved residues to carboxylation, we replaced FX propeptide sequence between -11 and -18 with that of PC (FX/PC1) (Figure 3B), a propeptide that has approximately 90-fold lower affinity for GGCX.16 Results from our cell- based study show that this replacement does not show an obvious effect on reporter-protein carboxylation (Figure 3D). However, when we exchanged the propeptide sequence between -5 and -9 (FX/PC2) (Figure 3), reporter- protein carboxylation was increased approximately 3- fold. Further replacement of FX propeptide between -5 and -18 with that of PC (FX/PC3) (Figure 3), increased reporter-protein carboxylation approximately 6-fold, a level similar to the full-length propeptide of PC (Figure 3D). These results suggest that the entire N-terminal sequence of the propeptide (-5 to -18) determines the car- boxylation efficiency of coagulation factors, which is con- sistent with previous observations.13
haematologica | 2020; 105(8)
2167