Page 79 - Haematologica - Vol. 105 n. 6 - June 2020
P. 79
Innate drug responses in hematologic cell populations
AB
CD
E
F
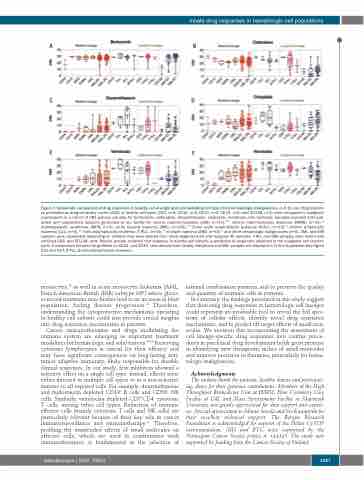
Figure 7. Systematic comparison of drug responses in healthy cell-of-origin and corresponding cell types from hematologic malignancies. (A-F) Ex vivo drug respons- es presented as drug sensitivity scores (DSS) of healthy cell types (CD3, n=4; CD14, n=4; CD19, n=2; CD34, n=2; and CD138, n=3) were compared to malignant counterparts in a cohort of 281 primary samples for bortezomib, clofarabine, dexamethasone, omipalisib, venetoclax and navitoclax. Samples included both pub- lished and unpublished datasets generated at our facility for chronic myeloid leukemia (CML) (n=13),10,11 chronic myelomonocytic leukemia (CMML) (n=11),12 myelodysplastic syndromes (MDS) (n=4), acute myeloid leukemia (AML) (n=145),9,12 B-cell acute lymphoblastic leukemia (B-ALL) (n=14),13 chronic lymphocytic leukemia (CLL) (n=4),12 T-cell prolymphocytic leukemia (T-PLL) (n=40),14 multiple myeloma (MM) (n=50)15 and other hematologic malignancies (n=6). AML and MM samples were subdivided depending on whether they were derived from newly diagnosed (D) and relapsed (R) samples. T-PLL and MM samples were tested with enriched CD8+ and CD138+ cells. Results provide evidence that response in healthy cell subsets is predictive of responses observed in the malignant cell counter- parts. A comparison between drug effects on CD14+ and CD34+ cells derived from healthy individuals and AML samples are displayed in Online Supplementary Figure S13 and S14. B-PLL: B-cell prolymphocytic leukemia.
monocytes,43 as well in acute monocytic leukemia [AML, French-American-British (FAB) subtype M5] where gluco- corticoid treatment may further lead to an increase in blast population, fueling disease progression.44 Therefore, understanding the cytoprotective mechanisms operating in healthy cell subsets could also provide crucial insights into drug resistance mechanisms in patients.
Cancer immunotherapies and drugs modulating the immune system are emerging as important treatment modalities for hematologic and solid tumors.45,46 Preserving cytotoxic lymphocytes is critical for their efficacy and may have significant consequences on long-lasting anti- tumor adaptive immunity, likely responsible for durable clinical responses. In our study, few inhibitors showed a selective effect on a single cell type; instead, effects were either directed to multiple cell types or in a non-selective manner to all exposed cells. For example, dexamethasone and midostaurin depleted CD19+ B cells and CD56+ NK cells. Similarly, venetoclax depleted CD3+CD4- cytotoxic T cells, among other cell types. Reduction of immune effector cells (mainly cytotoxic T cells and NK cells) are particularly relevant because of their key role in cancer immunosurveillance and immunotherapy.47 Therefore, profiling the unintended effects of small molecules on effector cells, which are used in combination with immunotherapies, is fundamental to the selection of
rational combination partners, and to preserve the quality and quantity of immune cells in patients.
In summary, the findings presented in this study suggest that dissecting drug responses in hematologic cell lineages could represent an invaluable tool to reveal the full spec- trum of cellular effects, identify novel drug resistance mechanisms, and to predict off target effects of small mol- ecules. We envision that incorporating the assessment of cell lineage-specific drug responses into routine proce- dures in preclinical drug development holds great promise in identifying new therapeutic niches of small molecules and improve precision in therapies, particularly for hema- tologic malignancies.
Acknowledgments
The authors thank the patients, healthy donors and participat- ing clinics for their generous contributions. Members of the High Throughput Biomedicine Unit at FIMM, Flow Cytometry Core Facility at UiB, and Mass Spectrometry Facility at Maynooth University, are greatly appreciated for their support and expert- ise. Special appreciation to Minna Suvela and Siv Knaappila for their excellent technical support. The Bergen Research Foundation is acknowledged for support of the Helios CyTOF instrumentation. MH and BTG were supported by the Norwegian Cancer Society project n. 144345. The study was supported by funding from the Cancer Society of Finland.
haematologica | 2020; 105(6)
1537