Page 78 - Haematologica - Vol. 105 n. 6 - June 2020
P. 78
M.M. Majumder et al.
C
DE
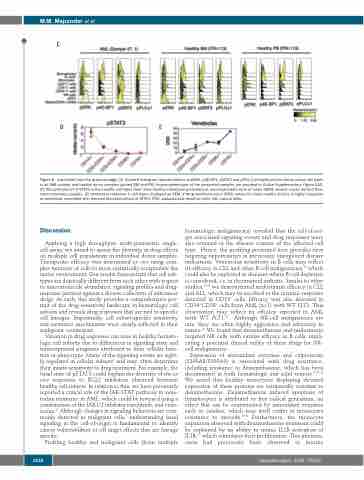
Figure 6. (continued from the previous page) (C) Stacked histogram representations of pERK, p4E-BP1, pSTAT3 and pPLCγ1 phosphorylation status across cell types in an AML patient and healthy donor samples (paired BM and PB). Immunophenotype of the presented samples are provided in Online Supplementary Figure S1B. (D) Phosphorylation of STAT3 in five healthy cell types from three healthy individuals presented as mean±standard error of mean (SEM) arcsinh values derived from mass cytometry analysis. (E) Venetoclax response in cell types displayed as SEM of drug sensitivity score (DSS) values for three healthy donors. A higher response to venetoclax correlated with reduced phosphorylation of STAT3. PDC: plasmacytoid dendritic cells; NK: natural killer.
Discussion
Applying a high throughput, multi-parametric single- cell assay, we aimed to assess the diversity in drug effects on multiple cell populations in individual donor samples. Therapeutic efficacy was determined ex vivo using com- plex mixtures of cells to more realistically recapitulate the native environment. Our results demonstrate that cell sub- types are drastically different from each other with respect to macromolecule abundance, signaling profiles and drug- response patterns against a diverse collection of anticancer drugs. As such, this study provides a comprehensive por- trait of the drug sensitivity landscape in hematologic cell subsets and reveals drug responses that are tied to specific cell lineages. Importantly, cell subset-specific sensitivity and resistance mechanisms were clearly reflected in their malignant counterpart.
Variation in drug responses can arise in healthy hemato- logic cell subsets due to differences in signaling state and transcriptional programs attributed to their cellular func- tion or phenotype. Many of the signaling events are tight- ly regulated in cellular subsets2 and may often determine their innate sensitivity to drug treatment. For example, the basal state of pSTAT3 could explain the diversity of the ex vivo responses to BCL2 inhibitors observed between healthy cell subsets. In relation to this, we have previously reported a critical role of the JAK-STAT pathway in vene- toclax resistance in AML, which could be reversed using a combination of the JAK1/2 inhibitor ruxolitinib, and vene- toclax.32 Although changes in signaling behaviors are com- monly detected in malignant cells,7 understanding basal signaling in the cell-of-origin is fundamental to identify cancer vulnerabilities or off target effects that are lineage specific.
Profiling healthy and malignant cells (from multiple
hematologic malignancies) revealed that the cell-of-ori- gin associated signaling events and drug responses were also retained in the disease context of the affected cell type. Hence, the profiling presented here provides new targeting opportunities in previously unexplored disease indications. Venetoclax sensitivity in B cells may reflect its efficacy in CLL and other B-cell malignancies,33 which could also be exploited in diseases where B-cell depletion is considered, i.e. in rheumatoid arthritis. Similar to other studies,34,35 we demonstrated midostaurin efficacy in CLL and ALL, which may be ascribed to the intrinsic response detected in CD19+ cells. Efficacy was also detected in CD34+CD38+ cells from AML (n=1) with WT FLT3. This observation may reflect its efficacy reported in AML with WT FLT3.24 Although NK-cell malignancies are rare, they are often highly aggressive and refractory in nature.36 We found that dexamethasone and midostaurin targeted NK cells with similar efficacy as B cells, impli- cating a potential clinical utility of these drugs for NK- cell malignancies.
Expression of antioxidant enzymes and calprotectin (S100A8/S100A9) is associated with drug resistance, including resistance to dexamethasone, which has been documented in both hematologic and solid tumors.29,37-40 We noted that healthy monocytes displaying elevated expression of these proteins are intrinsically resistant to dexamethasone. Dexamethasone induced apoptosis of lymphocytes is attributed to free radical generation, an effect that can be counteracted by antioxidant enzymes such as catalase, which may itself confer in monocytes resistance to steroids.30,41 Furthermore, the monocyte expansion observed with dexamethasone treatment could be explained by its ability to mimic IL1B activation of IL1R,42 which stimulates their proliferation. This phenom- enon had previously been observed in murine
1536
haematologica | 2020; 105(6)