Page 38 - Haematologica - Vol. 105 n. 6 - June 2020
P. 38
G. Pavlasova and M. Mraz
order to fully exploit their therapeutic potential. This is underscored by the recent disappointing results of clinical trials testing rituximab’s addition to the BTK inhibitor ibrutinib in CLL, which showed practically no benefit of such a combination.12 Here we summarize the research describing the regulation and function of CD20 in normal and malignant B cells, and the therapeutic implications of these observations, including the relevance for the combi- nation of “BCR inhibitors” with anti-CD20 monoclonal antibodies.
CD20 gene and protein structure
CD20 is a 33-37 kDa non-glycosylated protein expressed on the surface of normal and malignant B lym- phocytes, and belongs to the MS4A (membrane-spanning 4-domain family A) protein family.13 To date, 18 MS4A family members have been identified, besides MS4A1 (encoding CD20), also the high-affinity immunoglobulin E receptor β subunit (MS4A2/FcεRIβ) or HtM4 gene (MS4A3) (reviewed by Eon Kuek14). MS4A proteins are transmembrane molecules and they are predicted to share a similar polypeptide sequence and overall topological structure. The majority of MS4A genes, including MS4A1, are localized within a cluster on chromosome 11q12 in humans (chromosome 19 in mice), and two members
from a closely related TMEM176 gene family were identi- fied in chromosome region 7q36.1.14
The MS4A1 gene is 16 kb long, comprises eight exons, and several different CD20 mRNA transcripts have been annotated.13 The dominant CD20 mRNA variant is 2.8 kb long and uses all eight exons, whereas the second most common form is 263 bases shorter, as it skips exon II. A minor 3.5 kb mRNA results from splicing exons in the upstream region into an internal 3' splice site located in exon I. However, all three transcripts are translated into identical full-length CD20 protein as the translation start codon is localized within exon III. Moreover, other alter- native transcripts were identified in malignant B cells, some of them encoding truncated forms of CD20 protein leading to impaired binding of anti-CD20 monoclonal antibodies.15,16
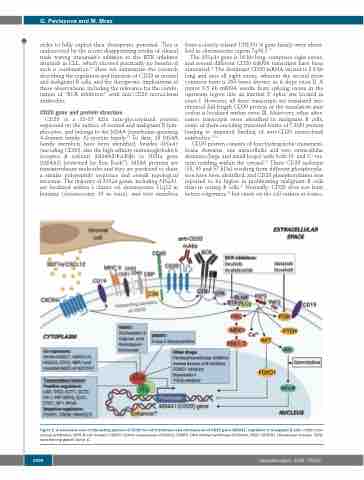
CD20 protein consists of four hydrophobic transmem- brane domains, one intracellular and two extracellular domains (large and small loops) with both N- and C- ter- mini residing within the cytosol.14 Three CD20 isoforms (33, 35 and 37 kDa) resulting from different phosphoryla- tion have been identified, and CD20 phosphorylation was reported to be higher in proliferating malignant B cells than in resting B cells.17 Normally, CD20 does not form hetero-oligomers,18 but exists on the cell surface as homo-
Figure 2. A schematic view of interacting partners of CD20 on cell membrane and mechanisms of CD20 gene (MS4A1) regulation in malignant B cells. mAbs: mon- oclonal antibodies; BCR: B-cell receptor; HDACi: histone deacetylase inhibitors; DNMTi: DNA methyl-transferase inhibitors; NICD: NOTCH1 intracellular domain; TGFβ: transforming growth factor β.
1496
haematologica | 2020; 105(6)