Page 37 - Haematologica - Vol. 105 n. 6 - June 2020
P. 37
CD20: functions and regulation
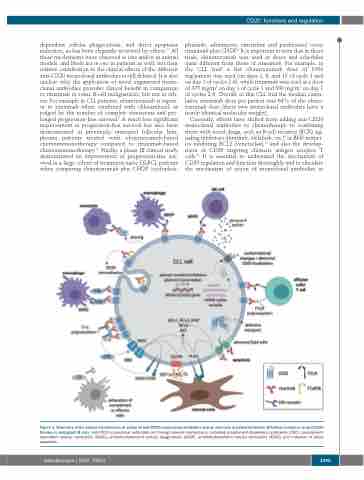
dependent cellular phagocytosis, and direct apoptosis induction, as has been elegantly reviewed by others.3,4 All these mechanisms were observed in vitro and/or in animal models, and likely act in vivo in patients as well, but their relative contribution to the clinical effects of the different anti-CD20 monoclonal antibodies is still debated. It is also unclear why the application of novel engineered mono- clonal antibodies provides clinical benefit in comparison to rituximab in some B-cell malignancies, but not in oth- ers. For example in CLL patients, obinutuzumab is superi- or to rituximab when combined with chlorambucil, as judged by the number of complete remissions and pro- longed progression-free survival.5 A much less significant improvement in progression-free survival has also been demonstrated in previously untreated follicular lym- phoma patients treated with obinutuzumab-based chemoimmunotherapy compared to rituximab-based chemoimmunotherapy.6,7 Finally, a phase III clinical study demonstrated no improvement in progression-free sur- vival in a large cohort of treatment-naïve DLBCL patients when comparing obinutuzumab plus CHOP (cyclophos-
phamide, adriamycin, vincristine and prednisone) versus rituximab plus CHOP.8 It is important to note that in these trials, obinutuzumab was used at doses and schedules quite different from those of rituximab. For example, in the CLL trial5 a flat obinutuzumab dose of 1000 mg/patient was used (on days 1, 8, and 15 of cycle 1 and on day 1 of cycles 2-6), while rituximab was used at a dose of375mg/m2 onday1ofcycle1and500mg/m2 onday1 of cycles 2-6. Overall, in this CLL trial the median cumu- lative rituximab dose per patient was 64% of the obinu- tuzumab dose (these two monoclonal antibodies have a nearly identical molecular weight).
Currently, efforts have shifted from adding anti-CD20 monoclonal antibodies to chemotherapy to combining them with novel drugs, such as B-cell receptor (BCR) sig- naling inhibitors (ibrutinib, idelalisib, etc.)9 or BH3-mimet- ics inhibiting BCL2 (venetoclax),10 and also the develop- ment of CD20 targeting chimeric antigen receptor T cells.11 It is essential to understand the mechanism of CD20 regulation and function thoroughly and to elucidate the mechanism of action of monoclonal antibodies in
Figure 1. Summary of the known mechanisms of action of anti-CD20 monoclonal antibodies and an overview of potential factors affecting resistance to anti-CD20 therapy in malignant B cells. Anti-CD20 monoclonal antibodies act through several mechanisms, including complement-dependent cytotoxicity (CDC), complement- dependent cellular cytotoxicity (CDCC), antibody-dependent cellular phagocytosis (ADCP), antibody-dependent cellular cytotoxicity (ADCC), and induction of direct apoptosis.
haematologica | 2020; 105(6)
1495