Page 243 - Haematologica - Vol. 105 n. 6 - June 2020
P. 243
The FVIII binding site on VWF
interacts with FVIII rather than being important for stabi- lizing the local conformation. Th replacement of Leu786 by an alanine, most likely, alters the conformation of the short helical region 786-Leu-Glu-Cys-789 thereby reposi- tioning Glu787 (Figure 5A). This can explain, in our view,
the altered FVIII binding efficiency of the Leu786Ala vari- ant (Figure 5B-C). HDX-MS did not reveal reduced deu- terium incorporation in peptides that include Lys773. This residue is part of a beta-sheet in which the amino back- bone hydrogens tightly interact. Apparently, this second-
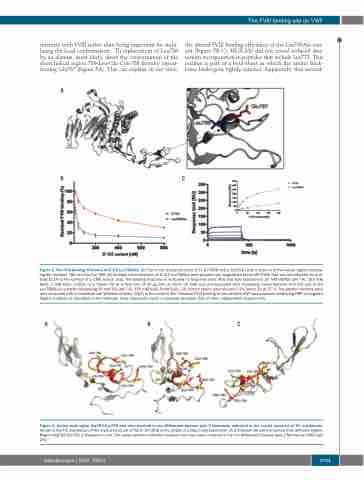
A
BC
Figure 5. The FVIII binding efficiency of D’-D3 Leu786Ala. (A) Part of the crystal structure of D’-D3 (PDB entry: 6n29)30 with a zoom-in of the helical region compris- ing the residues 786-Leu-Glu-Cys-789. (B) Multiple concentrations of D’-D3 Leu786Ala were passed over coagulation factor VIII (FVIII) that was immobilized via anti- body EL14 to the surface of a CM5 sensor chip. The binding response is indicated as response units (RU) and was assessed in 20 mM HEPES (pH 7.4), 150 mM NaCl, 5 mM CaCl2, 0.05% (v/v) Tween 20 at a flow rate of 30 μL/min at 25oC. (C) FVIII was pre-incubated with increasing concentrations of D’-D3 and D’-D3 Leu786Ala in a buffer comprising 50 mM Tris (pH 7.4), 150 mM NaCl, 5mM CaCl2, 2% human serum albumin and 0.1% Tween 20 at 37˚C. The protein mixtures were next incubated with immobilized von Willebrand factor (VWF) in the same buffer. Residual FVIII binding to immobilized VWF was assessed employing HRP-conjugated CAg12 antibody as described in the methods. Data represents mean ± standard deviation (SD) of three independent experiments.
Figure 6. Amino acid region Arg782-Cys799 and sites involved in von Willebrand disease type 2 Normandy indicated in the crystal structure of TIL’ subdomain.
Shown is the TIL’ subdomain of the crystal structure of the D’-D3 (PDB entry: 6n29) in a ribbon representation. (A-C) Present the same structure from different angels. Region Arg782-Cys799 is displayed in red. The yellow spheres indicated residues that have been mutated in the von Willebrand disease type 2 Normandy (VWD type 2N).37
ABC
haematologica | 2020; 105(6)
1701