Page 242 - Haematologica - Vol. 105 n. 6 - June 2020
P. 242
M. A.Przeradzka et al.
in the region Arg782-Cys799 are involved in FVIII binding. In particular, the glutamic acid residue at position 787 seems critical for the interaction between FVIII and D’-D3.
A leucine at position 786 is important for effective interaction with FVIII
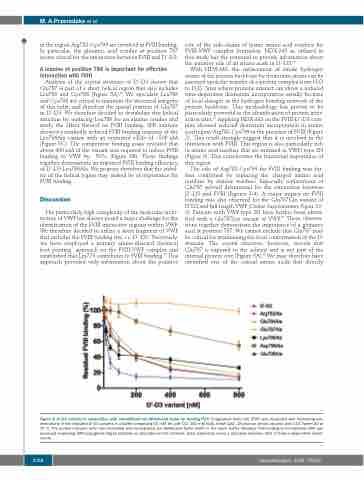
Analysis of the crystal structure of D’-D3 shows that Glu787 is part of a short helical region that also includes Leu786 and Cys788 (Figure 5A).30 We speculate Leu786 and Cys788 are critical to maintain the structural integrity of this helix, and therefore the spatial position of Glu787 in D’-D3. We therefore decided to destabilize this helical structure by replacing Leu786 for an alanine residue and study the effect thereof on FVIII binding. SPR analysis showed a markedly reduced FVIII binding response of the Leu786Ala variant with an estimated <Kd> of ~500 nM (Figure 5C). The competitive binding assay revealed that about 400 nM of the variant was required to reduce FVIII binding to VWF by 50% (Figure 5B). These findings together demonstrate an impaired FVIII binding efficiency of D’-D3 Leu786Ala. We propose therefore that the stabil- ity of the helical region may indeed be of importance for FVIII binding.
Discussion
The particularly high complexity of the molecular archi- tecture of VWF has always posed a major challenge for the identification of the FVIII interactive regions within VWF. We therefore decided to utilize a short fragment of VWF that includes the FVIII binding site, i.e. D’-D3.2 Previously, we have employed a primary amine-directed chemical foot printing approach on the FVIII-VWF complex and established that Lys773 contributes to FVIII binding.23 This approach provided only information about the putative
role of the side-chains of lysine amino acid residues for FVIII-VWF complex formation. HDX-MS as utilized in this study has the potential to provide information about the putative role of all amino acids in D’-D3.33
With HDX-MS, the replacement of amide hydrogen atoms of the protein backbone by deuterium atoms can be assessed upon the transfer of a protein complex from H2O to D2O. Sites where proteins interact can show a reduced time-dependent deuterium incorporation usually because of local changes in the hydrogen bonding network of the protein backbone. This methodology has proven to be particularly powerful in the identification of protein inter- action sites.34 Applying HDX-MS on the FVIII-D’-D3 com- plex showed reduced deuterium incorporation in amino acid region Arg782-Cys799 in the presence of FVIII (Figure 2). This result strongly suggest that it is involved in the interaction with FVIII. This region is also particularly rich in amino acid residues that are mutated in VWD type 2N (Figure 6). This corroborates the functional importance of this region.
The role of Arg782-Cys799 for FVIII binding was fur- ther confirmed by replacing the charged amino acid residues by alanine residues. Especially replacement of Glu787 proved detrimental for the interaction between D’-D3 and FVIII (Figures 3-4). A major impact on FVIII binding was also observed for the Glu787Gln variant of D’D3 and full-length VWF (Online Supplementary Figure S2- 3). Patients with VWF type 2N have further been identi- fied with a Glu787Lys variant of VWF.35 These observa- tions together demonstrate the importance of a glutamic acid at position 787. We cannot exclude that Glu787 may be critical for maintaining the local conformation of the D’ domain. The crystal structure, however, reveals that Glu787 is exposed to the solvent and is not part of the internal protein core (Figure 5A).30 We may therefore have identified one of the critical amino acids that directly
Figure 4. D’-D3 variants in competition with immobilized von Willebrand factor for binding FVIII. Coagulation factor VIII (FVIII) was incubated with increasing con- centrations of the indicated D’-D3 variants in a buffer comprising 50 mM Tris (pH 7.4), 150 mM NaCl, 5mM CaCl2, 2% human serum albumin and 0.1% Tween 20 at 37˚C. The protein mixtures were next incubated with immobilized von Willebrand factor (VWF) in the same buffer. Residual FVIII binding to immobilized VWF was assessed employing HRP-conjugated CAg12 antibody as described in the methods. Data represents mean ± standard deviation (SD) of three independent experi- ments.
1700
haematologica | 2020; 105(6)