Page 240 - Haematologica - Vol. 105 n. 6 - June 2020
P. 240
M. A.Przeradzka et al.
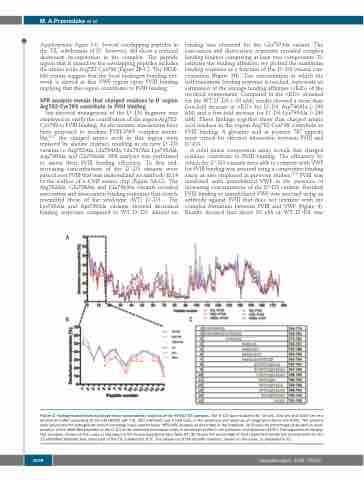
Supplementary Figure S1). Several overlapping peptides in the TIL’ subdomain of D’, however, did show a reduced deuterium incorporation in the complex. The peptide region that is shared by the overlapping peptides includes the amino acids Arg782-Cys799 (Figure 2B-C). The HDX- MS results suggest that the local hydrogen bonding net- work is altered in this VWF region upon FVIII binding implying that this region contributes to FVIII binding.
SPR analysis reveals that charged residues in D’ region Arg782-Cys799 contribute to FVIII binding
Site-directed mutagenesis of the D’-D3 fragment was employed to verify the contribution of the region Arg782- Cys799 to FVIII binding. As electrostatic interactions have been proposed to mediate FVIII-VWF complex assem- bly,31,32 the charged amino acids in this region were replaced by alanine residues resulting in six new D’-D3 variants i.e. Arg782Ala, Glu784Ala, Glu787Ala, Lys790Ala, Asp796Ala and Glu798Ala. SPR analysis was performed to assess their FVIII binding efficiency. To this end, increasing concentrations of the D’-D3 variants were passed over FVIII that was immobilized via antibody EL14 to the surface of a CM5 sensor chip (Figure 3A-G). The Arg782Ala, Glu784Ala and Glu798Ala variants revealed association and dissociation binding responses that closely resembled those of the wild-type (WT) D’-D3. The Lys790Ala and Aps796Ala variants showed decreased binding responses compared to WT D’-D3. Almost no
binding was observed for the Glu787Ala variant. The association and dissociation responses revealed complex binding kinetics comprising at least two components. To estimate the binding affinities, we plotted the maximum binding response as a function of the D’-D3 variant con- centration (Figure 3H). The concentration at which the half-maximum binding response is reached, represents an estimation of the average binding affinities (<Kd>) of the involved components. Compared to the <KD> obtained for the WT D’-D3 (~50 nM), results showed a more than four-fold increase in <KD> for D’-D3 Asp796Ala (~190 nM) and a five-fold increase for D’-D3 Lys790Ala (~240 nM). These findings together show that charged amino acid residues in the region Arg782-Cys799 contribute to FVIII binding. A glutamic acid at position 787 appears most critical for effective interaction between FVIII and D’-D3.
A solid phase competition assay reveals that charged residues contribute to FVIII binding. The efficiency by which the D’-D3 variants were able to compete with VWF for FVIII binding was assessed using a competitive binding assay as also employed in previous studies.22,23 FVIII was incubated with immobilized VWF in the presence of increasing concentrations of the D’-D3 variants. Residual FVIII binding to immobilized VWF was assessed using an antibody against FVIII that does not interfere with the complex formation between FVIII and VWF (Figure 4). Results showed that about 50 nM of WT D’-D3 was
A
BC
Figure 2. Hydrogen-deuterium exchange mass spectrometry analysis of the FVIII-D’-D3 complex. The D’-D3 was incubated for 10 sec, 100 sec and 1000 sec in a deuterium buffer consisting of 20 mM HEPES (pH 7.4), 150 mM NaCl and 5 mM CaCl2 in the presence and absence of coagulation factor VIII (FVIII). The proteins were processed for hydrogen-deuterium exchange mass spectrometry (HDX-MS) analysis as described in the methods. (A) Shows the percentage of deuterium incor- poration of the identified peptides of the D’-D3 at the indicated incubation times in deuterium buffer in the presence and absence of FVIII. The sequence of the pep- tide numbers, shown on the x-axis, is displayed in the Online Supplementary Table S1. (B) Shows the percentage of time-dependent deuterium incorporation for the 15 identified peptides that cover part of the TIL’ subdomain of D’. The sequence of the peptide numbers, shown on the x-axis, in displayed in (C).
1698
haematologica | 2020; 105(6)