Page 187 - Haematologica - Vol. 105 n. 6 - June 2020
P. 187
PIKfyve inhibition in multiple myeloma
AB
CD
E
F
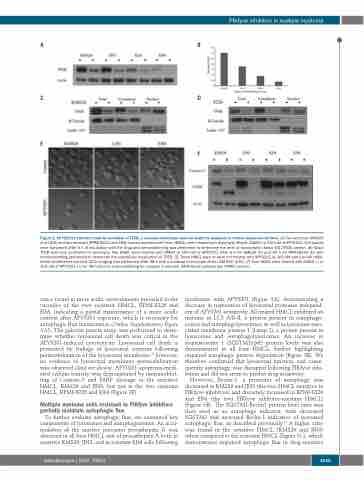
Figure 2. APY0201 treatment leads to activation of TFEB, a vacuolar phenotype, and cell death by apoptosis in human myeloma cell lines. (A) Two sensitive (KMS26 and JJN3) and two resistant (RPMI-8226 and EJM) human myeloma cell lines (HMCL) were treated with dimethylsulfoxide (DMSO) or 100 nM of APY0201. Cell lysates were harvested after 6 h of incubation with the drug and immunoblotting was performed to determine the level of transcription factor EB (TFEB) protein. (B) Basal TFEB level was correlated to sensitivity. Two HMCL were treated with DMSO or 100 nM of APY0201 after 6 h for KMS26 (C) and 24 h for RPMI-8226 (D) with immunoblotting performed to determine the subcellular localization of TFEB. (E) Three HMCL were or were not treated with APY0201 at 100 nM and live cell differ- ential interference contrast (DIC) imaging was performed after 48 h with a confocal microscope Zeiss LSM 800 (63X). (F) Four HMCL were treated with DMSO (-) or 100 nM of APY0201 (+) for 48 h prior to immunoblotting for caspase-3 and poly (ADP-ribose) polymerase (PARP) protein.
cence found in more acidic environments prevailed in the vacuoles of the two resistant HMCL, RPMI-8226 and EJM, indicating a partial maintenance of a more acidic content after APY0201 exposure, which is necessary for autophagic flux maintenance (Online Supplementary Figure S10). The galectin puncta assay was performed to deter- mine whether lysosomal cell death was critical in the APY0201-induced cytotoxicity. Lysosomal cell death is promoted by leakage of lysosomal contents following permeabilization of the lysosomal membrane;32 however, no evidence of lysosomal membrane permeabilization was observed (data not shown). APY0201 apoptosis-medi- ated cellular toxicity was demonstrated by immunoblot- ting of Caspase-3 and PARP cleavage in the sensitive HMCL, KMS26 and JJN3, but not in the two resistant HMCL, RPMI-8226 and EJM (Figure 2F).
Multiple myeloma cells resistant to PIKfyve inhibitors partially maintain autophagic flux
To further evaluate autophagic flux, we examined key components of lysosomes and autophagosomes. An accu- mulation of the inactive precursor procathepsin D was observed in all four HMCL and of procathepsin A both in sensitive KMS26, JJN3, and in resistant EJM cells following
incubation with APY0201 (Figure 3A), demonstrating a decrease in maturation of lysosomal proteases independ- ent of APY0201 sensitivity. All treated HMCL exhibited an increase in LC3 A/B-II, a protein present in autophago- somes and autophagolysosomes; as well as lysosome-asso- ciated membrane protein 1 (Lamp-1), a protein present in lysosomes and autophagolysosomes. An increase in sequestosome 1 (SQSTM1)/p62 protein levels was also demonstrated in all four HMCL, further highlighting impaired autophagic protein degradation (Figure 3B). We therefore confirmed that lysosomal function, and conse- quently autophagy, was disrupted following PIKfyve inhi- bition and did not seem to predict drug sensitivity.
However, Beclin-1, a promoter of autophagy was decreased in KMS26 and JJN3 (the two HMCL sensitive to PIKfyve inhibitors) and discretely increased in RPMI-8226 and EJM (the two PIKfyve inhibitor-resistant HMCL) (Figure 3B). The SQSTM1:Beclin1 protein level ratio was then used as an autophagy indicator, with decreased SQSTM1 and increased Beclin-1 indicative of increased autophagic flux, as described previously.33 A higher ratio was found in the sensitive HMCL (KMS26 and JJN3) when compared to the resistant HMCL (Figure 3C), which demonstrates impaired autophagic flux in drug-sensitive
haematologica | 2020; 105(6)
1645