Page 168 - Haematologica - Vol. 105 n. 6 - June 2020
P. 168
E.C. Rotbain et al.
whether the treatment regimens were pooled or separate [pooled: HR=1.15 (95% CI: 0.80-1.66), FCR-treated: HR=1.21 (95% CI: 0.53-2.77), BR-treated: HR=0.78 (95% CI: 0.14-4.15), CD20-chlorambucil-treated: HR=0.86 (95% CI: 0.38-1.91) and chlorambucil-treated: HR=1.31 (95% CI: 0.75-2.28)].
Treatment-free survival after first-line treatment
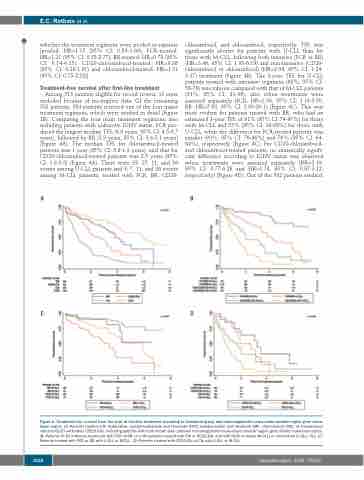
Among 513 patients eligible for record review, 11 were excluded because of incomplete data. Of the remaining 502 patients, 384 patients received one of the four major treatment regimens, which were studied in detail (Figure 1B). Comparing the four main treatment regimens, also including patients with unknown IGHV status, FCR pro- duced the longest median TFSt (6.0 years, 95% CI: 4.5-6.7 years), followed by BR (3.9 years, 95% CI: 3.4-5.1 years) (Figure 4A). The median TFSt for chlorambucil-treated patients was 1 year (95% CI: 0.8-1.3 years), and that for CD20-chlorambucil-treated patients was 2.5 years (95% CI: 1.8-3.3) (Figure 4A). There were 39, 27, 11, and 36 events among U-CLL patients and 9, 7, 11, and 26 events among M-CLL patients, treated with FCR, BR, CD20-
chlorambucil, and chlorambucil, respectively. TFSt was significantly shorter for patients with U-CLL than for those with M-CLL, following both intensive (FCR or BR) (HR=3.46, 95% CI: 1.93-6.19) and non-intensive (CD20- chlorambucil or chlorambucil) (HR=2.04, 95% CI: 1.24- 3.37) treatment (Figure 4B). The 3-year TFSt for U-CLL patients treated with intensive regimens (68%, 95% CI: 58-76) was inferior compared with that of M-CLL patients (91%, 95% CI: 81-96), also when treatments were assessed separately (FCR: HR=2.56, 95% CI: 1.19-5.50; BR: HR=7.50, 95% CI: 2.80-20.1) (Figure 4C). This was most evident for patients treated with BR, who had an estimated 3-year TFSt of 91% (95% CI: 74-97%) for those with M-CLL and 53% (95% CI: 36-68%) for those with U-CLL, while the difference for FCR-treated patients was smaller (90%, 95% CI: 76-96%) and 76% (95% CI: 64- 84%), respectively (Figure 4C). For CD20-chlorambucil- and chlorambucil-treated patients, no statistically signifi- cant difference according to IGHV status was observed when treatments were assessed separately (HR=2.19, 95% CI: 0.77-6.28 and HR=1.74, 95% CI: 0.97-3.12, respectively) (Figure 4D). Out of the 502 patients studied,
AB
CD
Figure 4. Treatment-free survival from the start of first-line treatment according to treatment group and immunoglobulin heavy-chain variable region gene muta- tional status. (A) Patients treated with fludarabine, cyclophosphamide and rituximab (FCR), bendamustine and rituximab (BR), chlorambucil (Clb), or chlorambucil and anti-CD20 antibodies (CD20-Clb), including patients with both known and unknown immunoglobulin heavy-chain variable region gene (IGHV) mutational status. (B) Patients fit for intensive treatment with FCR of BR, or unfit patients treated with Clb or CD20-Clb, and with IGHV mutated (M-CLL) or unmutated (U-CLL) CLL. (C) Patients treated with FCR or BR with U-CLL or M-CLL. (D) Patients treated with CD20-Clb or Clb with U-CLL or M-CLL.
1626
haematologica | 2020; 105(6)