Page 166 - Haematologica - Vol. 105 n. 6 - June 2020
P. 166
E.C. Rotbain et al.
CLL, 263 (12%) patients with M-CLL, and 227 (24%) patients with unknown IGHV status died without receiv- ing CLL treatment, while the numbers of events for OSd, and TFSd, were, respectively, 263 and 573 for patients with U-CLL, 384 and 632 for patients with M-CLL, and 360 and 482 for patients with unknown IGHV status.
Overall survival after first-line treatment
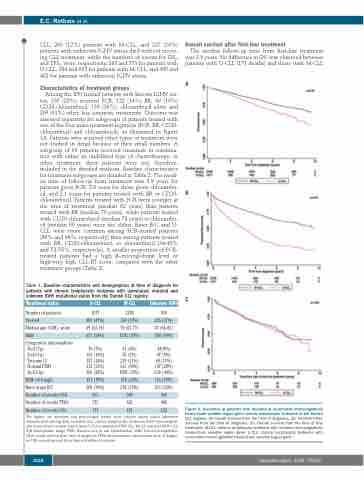
The median follow-up time from first-line treatment was 2.9 years. No difference in OSt was observed between patients with U-CLL (171 deaths) and those with M-CLL
A
B
C
Figure 2. Outcomes of patients with mutated or unmutated immunoglobulin heavy-chain variable region gene chronic lymphocytic leukemia in the Danish CLL registry. (A) Overall survival from the time of diagnosis. (B) Treatment-free survival from the time of diagnosis. (C). Overall survival from the time of first treatment. M-CLL: chronic lymphocytic leukemia with mutated immunoglobulin heavy-chain variable region gene; U-CLL: chronic lymphocytic leukemia with unmutated immunoglobulin heavy-chain variable region gene.
Characteristics of treatment groups
Among the 850 treated patients with known IGHV sta- tus, 235 (28%) received FCR, 122 (14%) BR, 89 (10%) CD20-chlorambucil, 139 (16%), chlorambucil alone and 265 (31%) other, less common, treatments. Outcome was assessed separately for subgroups of patients treated with one of the four main treatment regimens (FCR, BR, CD20- chlorambucil and chlorambucil), as illustrated in Figure 1A. Patients who received other types of treatment were not studied in detail because of their small numbers. A subgroup of 99 patients received rituximab in combina- tion with either an undefined type of chemotherapy, or other treatment: these patients were not, therefore, included in the detailed analyses. Baseline characteristics for treatment subgroups are detailed in Table 2. The medi- an time of follow-up from treatment was 3.9 years for patients given FCR, 2.8 years for those given chlorambu- cil, and 2.1 years for patients treated with BR or CD20- chlorambucil. Patients treated with FCR were younger at the time of treatment (median 62 years) than patients treated with BR (median 70 years), while patients treated with CD20-chlorambucil (median 78 years) or chlorambu- cil (median 80 years) were the oldest. Binet B/C and U- CLL were more common among FCR-treated patients (56% and 64%, respectively) than among patients treated with BR, CD20-chlorambucil, or chlorambucil (34-42% and 52-58%, respectively). A smaller proportion of FCR- treated patients had a high β2-microglobulin level or high/very high CLL-IPI score, compared with the other treatment groups (Table 2).
Table 1. Baseline characteristics and demographics at time of diagnosis for patients with chronic lymphocytic leukemia with unmutated, mutated and unknown IGHV mutational status from the Danish CLL registry.
Mutational status
Number of patients
Treated
Median age (IQR), years Male
Cytogenetic abnormalities Del(17p)
Del(11q)
Trisomy 12
Normal FISH Del(13q)
B2M >4.0 mg/L
Binet stage B/C Number of events OSd Number of events TFSd
Number of events OSt
U-CLL
1017
481 (47%) 69 (62-76) 651 (64%)
70 (7%) 152 (16%) 157 (16%) 313 (33%) 269 (28%)
151 (19%)
308 (30%)
263
573
171
M-CLL
2180
369 (17%) 70 (63-77) 1292 (59%)
91 (4%)
50 (2%) 219 (11%) 613 (30%) 1088 (53%)
174 (10%)
292 (13%)
384
632
121
Unknown IGHV
936
255 (27%) 74 (66-82) 560 (60%)
44(8%) 47 (9%) 60 (11%) 147 (28%) 236 (44%)
116 (19%)
216 (23%)
360
482
133
The figures are numbers and percentages within each column unless stated otherwise. αPatients with missing data excluded. CLL: chronic lymphocytic leukemia; IGHV: immunoglob- ulin heavy-chain variable region gene; U-CLL: unmutated IGHV CLL; M-CLL: mutated IGHV CLL; IQR: interquartile range; FISH; fluorescence in situ hybridization; B2M: beta-2-microglobulin; OSd: overall survival from time of diagnosis; TFSd: treatment-free survival from time of diagno- sis; OSt: overall survival from time of first-line treatment.
1624
haematologica | 2020; 105(6)