Page 167 - Haematologica - Vol. 105 n. 6 - June 2020
P. 167
IGHV and outcome in CLL: a population-based study
(121 deaths) [3-year OSt 74% (95% CI: 70-78) and 72% (95% CI: 66-77), respectively] (Figure 2C). Patients with unknown IGHV status had an inferior OSt compared with U-CLL and M-CLL patients (133 deaths) [3-year OSt 59% (95% CI: 53- 65)] (data not shown). No impact on OSt was observed based on unmutated IGHV status (HR=0.99, 95% CI: 0.75-1.32), Binet stage B/C at diagnosis (HR 1.01, 95% CI: 0.76-1.35), or male sex (HR=0.97, 95% CI: 0.73- 1.29). Age above 65 years at the time of treatment (HR=3.18, 95% CI: 2.12- 4.77), high β2-microglobulin level (HR=1.92, 95% CI: 1.42-2.60), and del(17p) (HR=1.79,
95% CI: 1.21-2.65) were statistically significantly associat- ed with shorter OSt.
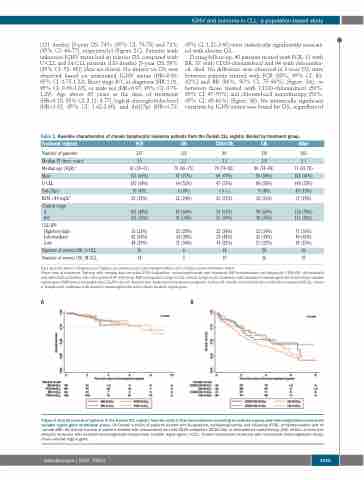
During follow-up, 40 patients treated with FCR, 11 with BR, 37 with CD20-chlorambucil and 94 with chlorambu- cil, died. No difference was observed in 3-year OSt rates between patients treated with FCR (88%, 95% CI: 83- 92%] and BR (86%, 95% CI: 75-93%) (Figure 3A), or between those treated with CD20-chlorambucil (59%, 95% CI: 47-70%) and chlorambucil monotherapy (53%, 95% CI: 45-61%) (Figure 3B). No statistically significant variation by IGHV-status was found for OSt, regardless of
Table 2. Baseline characteristics of chronic lymphocytic leukemia patients from the Danish CLL registry divided by treatment group.
Treatment regimen
Number of patients
Median FU time, yearsα Median age (IQR)α Male
U-CLL
Del(17p)β
B2M >4.0 mg/Lβ
Clinical stage A
B/C
CLL-IPIβ High/very high Intermediate Low
Number of events OSt, U-CLL
Number of events OSt, M-CLL
FCR
235
3.9
62 (55–67) 155 (66%) 150 (64%) 10 (4%) 25 (15%)
103 (44%)
132 (56%)
35 (21%) 82 (50%) 48 (29%)
26
14
BR
122
2.1
70 (66–75) 87 (71%) 64 (52%) 6 (6%) 22 (24%)
81 (66%)
41 (34%)
25 (28%) 34 (38%) 31 (34%)
6
5
CD20-Clb
89
2.1
78 (74–82) 60 (67%) 47 (53%) <5 (-) 20 (31%)
54 (61%)
35 (39%)
22 (34%) 28 (44%) 14 (22%)
18
19
Clb
139
2.8
80 (74–84) 80 (58%) 80 (58%) 9 (4%) 30 (31%)
90 (65%)
49 (35%)
32 (34%) 42 (44%) 21 (22%)
58
36
Other
265
3.1
71 (65-78) 162 (61%) 140 (53%) 40 (16%) 37 (18%)
154 (58%)
111 (42%)
71 (36%) 80 (41%) 45 (23%)
63
47
Data are from time of diagnosis, and figures are numbers and percentages within each column unless otherwise stated.
αFrom time of treatment. βPatients with missing data excluded. FCR, fludarabine, cyclophosphamide and rituximab; BR: bendamustine and rituximab; CD20-Clb: chlorambucil and anti-CD20 antibodies; Clb: chlorambucil; FU: follow-up; IQR: interquartile range; U-CLL: chronic lymphocytic leukemia with unmutated immunoglobulin heavy-chain variable region gene; B2M: beta-2-microglobulin; CLL-IPI: chronic lymphocytic leukemia international prognostic index; OSt: overall survival from time of first-line treatment; M-CLL: chron- ic lymphocytic leukemia with mutated immunoglobulin heavy-chain variable region gene.
AB
Figure 3. Overall survival of patients in the Danish CLL registry from the start of first-line treatment according to treatment group and immunoglobulin heavy-chain variable region gene mutational status. (A) Overall survival of patients treated with fludarabine, cyclophosphamide and rituximab (FCR), or bendamustine and rit- uximab (BR). (B) Overall survival of patients treated with chlorambucil and anti-CD20 antibodies (CD20-Clb) or chlorambucil monotherapy (Clb). M-CLL: chronic lym- phocytic leukemia with mutated immunoglobulin heavy-chain variable region gene; U-CLL: chronic lymphocytic leukemia with unmutated immunoglobulin heavy- chain variable region gene.
haematologica | 2020; 105(6)
1625