Page 153 - Haematologica - Vol. 105 n. 6 - June 2020
P. 153
KMT2D mutations and TP53 disruptions in MCL
AB
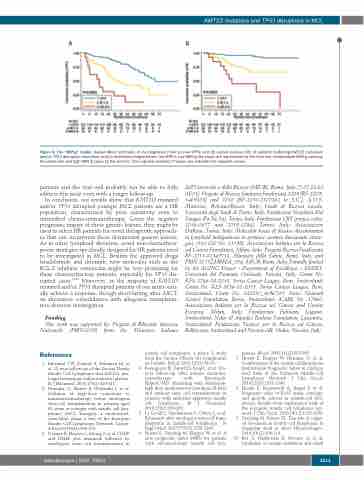
Figure 5. The “MIPI-g” model. Kaplan-Meier estimates of (A) progression free survival (PFS) and (B) overall survival (OS) of patients harboring KMT2D mutations and/or TP53 disruption (mutations and/or deletions) integrated into the MIPI-c. Low MIPI-g risk cases are represented by the blue line, intermediate MIPI-g cases by the yellow line and high MIPI-g cases by the red line. The Log-rank statistics P-values are indicated for adjacent curves.
patients and the trial will probably not be able to fully address this issue even with a longer follow-up.
In conclusion, our results show that KMT2D mutated and/or TP53 disrupted younger MCL patients are a HR population, characterized by poor sensitivity even to intensified chemo-immunotherapy. Given the negative prognostic impact of these genetic lesions, they might be used to select HR patients for novel therapeutic approach- es that can circumvent these detrimental genetic lesions. As in other lymphoid disorders, novel non-chemothera- peutic strategies specifically designed for HR patients need to be investigated in MCL. Besides the approved drugs lenalidomide and ibrutinib, new molecules such as the BCL-2 inhibitor venetoclax might be very promising for these chemorefractory patients, especially for TP53 dis- rupted cases.33,34 Moreover, as the majority of KMT2D mutated and/or TP53 disrupted patients of our series actu- ally achieve a response, though short-lasting after ASCT, an alternative consolidation with allogeneic transplanta- tion deserves investigation.
Funding
This work was supported by: Progetto di Rilevante Interesse Nazionale (PRIN2009) from the Ministero Italiano
dell'Università e della Ricerca (MIUR), Roma, Italy [7.07.02.60 AE01]; Progetto di Ricerca Sanitaria Finalizzata 2009 [RF-2009- 1469205] and 2010 [RF-2010-2307262 to S.C.], A.O.S. Maurizio, Bolzano/Bozen, Italy; Fondi di Ricerca Locale, Università degli Studi di Torino, Italy; Fondazione Neoplasie Del Sangue (Fo.Ne.Sa), Torino, Italy; Fondazione CRT (project codes: 2016.0677 and 2018.1284), Torino, Italy; Associazione DaRosa, Torino, Italy; Molecular bases of disease dissemination in lymphoid malignancies to optimize curative therapeutic strate- gies, (5x1,000 No. 21198), Associazione Italiana per la Ricerca sul Cancro Foundation, Milan, Italy; Progetto Ricerca Finalizzata RF-2011-02349712, Ministero della Salute, Rome, Italy; and PRIN 2015ZMRFEA_004, MIUR, Rome, Italy; Partially funded by the AGING Project – Department of Excellence – DIMET, Università del Piemonte Orientale, Novara, Italy; Grant No. KFS-3746-08-2015, Swiss Cancer League, Bern, Switzerland; Grant No. KLS-3636-02-2015, Swiss Cancer League, Bern, Switzerland; Grant No. 320030_169670/1 Swiss National Science Foundation, Berne, Switzerland; iCARE No. 17860, Associazione Italiana per la Ricerca sul Cancro and Unione Europea, Milan, Italy; Fondazione Fidinam, Lugano, Switzerland; Nelia & Amadeo Barletta Foundation, Lausanne, Switzerland; Fondazione Ticinese per la Ricerca sul Cancro, Bellinzona, Switzerland and Novara-AIL Onlus, Novara, Italy.
References
1. Eskelund CW, Kolstad A, Jerkeman M, et al. 15-year follow-up of the Second Nordic Mantle Cell Lymphoma trial (MCL2): pro- longed remissions without survival plateau. Br J Haematol. 2016;175(3):410-418.
2. Hermine O, Hoster E, Walewski J, et al. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lym- phoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet. 2016;388(10044):565-575.
3. Delarue R, Haioun C, Ribrag V, et al. CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in
mantle cell lymphoma: a phase 2 study from the Groupe d’Etude des Lymphomes de l’Adulte. Blood. 2013;121(1):48-53.
4. Romaguera JE, Fayad LE, Feng L, et al. Ten- year follow-up after intense chemoim- munotherapy with Rituximab- HyperCVAD alternating with Rituximab- high dose methotrexate/cytarabine (R-MA) and without stem cell transplantation in patients with untreated aggressive mantle cell lymphoma. Br J Haematol. 2010;150(2):200-208.
5. Le Gouill S, Thieblemont C, Oberic L, et al. Rituximab after autologous stem-cell trans- plantation in mantle-cell lymphoma. N Engl J Med. 2017;377(13):1250-1260.
6. Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lym-
phoma. Blood. 2008;111(2):558-565.
7. Hoster E, Klapper W, Hermine O, et al. Confirmation of the mantle-cell lymphoma International Prognostic Index in random- ized trials of the European Mantle-Cell Lymphoma Network. J Clin Oncol.
2014;32(13):1338-1346.
8. Hoster E, Rosenwald A, Berger F, et al.
Prognostic value of Ki-67 index, cytology, and growth pattern in mantle-cell lym- phoma: Results from randomized trials of the european mantle cell lymphoma net- work. J Clin Oncol. 2016;34(12):1386-1394.
9. Dreyling M, Ferrero SE. The role of target- ed treatment in mantle cell lymphoma: Is transplant dead or alive? Haematologica. 2016;101(2):104-114.
10. Beà S, Valdés-Mas R, Navarro A, et al. Landscape of somatic mutations and clonal
haematologica | 2020; 105(6)
1611