Page 68 - Haematologica May 2020
P. 68
A. Tichelli et al.
ry disease (n=8), or incomplete response (cyclosporine dependency, n=7; decreasing values but still in partial remission, n=8), 24 in the G-CSF group, and 16 in the non-G-CSF group (P=0.35). Sixteen patients needed a third-line therapy for relapse (n=5), refractory disease (n=7), or incomplete response (decreasing values but still in partial remission, n=4), 12 in the G-CSF group, and four in the non-G-CSF group (P=0.60). Twenty-eight patients needed allogeneic SCT as second-line or subse- quent treatment, 12 in the G-CSF group, and 16 in the non-G-CSF group, because of non-response/relapse (n=23), or MDS/AML (n=5). The cumulative probability of being treated with SCT at 15 years was 14±8% for patients who had been given G-CSF, and 22%±10% for those who had not (P=0.38) (Figure 3B). The 10-year sur- vival after transplantation was 63±18%.
Long-term follow-up
Next, we evaluated the probability of malignant and non-malignant late complications. During the follow-up, 52 of the 192 patients developed a late complication. Some of them developed more than one late event: 44 patients developed one, five developed two, and three developed three or more late complications. Nine patients developed clinical or morphological signs of MDS/AML, ten developed isolated cytogenetic abnormalities (2 cases with del(7q), 1 in the G-CSF group and 1 in the non-G- CSF group, and 1 case with each of the following abnor- malities: del(13q), del(9), loss of chromosome X in a female patient, loss of chromosome Y in a male patient, anomaly of chromosome 11 and translocation t(6;10); 2
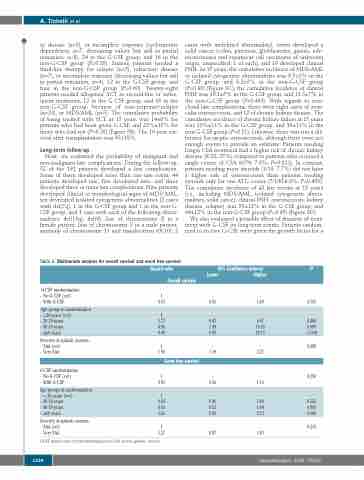
Table 2. Multivariate analysis for overall survival and event free-survival. Hazard ratio
cases with undefined abnormality), seven developed a solid cancer (colon, pancreas, glioblastoma, gastric, ade- nocarcinoma and squamous cell carcinoma of unknown origin, unspecified, 1 of each), and 19 developed clinical PNH. At 15 years, the cumulative incidence of MDS/AML or isolated cytogenetic abnormalities was 8.5±3% in the G-CSF group, and 8.2±3% in the non-G-CSF group (P=0.90) (Figure 3C); the cumulative incidence of clinical PNH was 10.1±5% in the G-CSF group, and 13.3±7% in the non-G-CSF group (P=0.499). With regards to non- clonal late complications, there were eight cases of avas- cular osteonecrosis, and 12 of chronic kidney disease. The cumulative incidence of chronic kidney failure at 15 years was 13%±11% in the G-CSF group, and 16±11% in the non-G-CSF group (P=0.51). Likewise, there was not a dif- ference for aseptic osteonecrosis, although there were not enough events to provide an estimate. Patients needing longer CSA treatment had a higher risk of chronic kidney disease (6/22; 27%) compared to patients who received a single course of CSA (6/79; 7.6%; P=0.021). In contrast, patients needing more steroids (1/13; 7.7%) did not have a higher risk of osteonecrosis than patients needing steroids only for one ATG course (7/100;6.2%; P=0.453). The cumulative incidence of all late events at 15 years (i.e., including MDS/AML, isolated cytogenetic abnor- malities, solid cancer, clinical PNH, osteonecrosis, kidney disease, relapse) was 50±12% in the G-CSF group, and 49±12% in the non-G-CSF group (P=0.65) (Figure 3D).
We also evaluated a possible effect of duration of treat- ment with G-CSF on long-term events. Patients random- ized to receive G-CSF, were given the growth factor for a
95% Confidence interval P Lower Higher
G-CSF randomization - No G-CSF (ref)
- With G-CSF
Age group at randomization - <20 years (ref)
- 20-39 years
- 40-59 years
Overall survival
1 --
0.91
1 1.77 4.96 9.08
1 1.95
Event free survival
1 0.81
1 0.83 0.93 1.61
1
1.27
0.55 1.49
0.703
- --
- ≥60 years
0.47 6.67 1.49 16.65 9.09 29.73
- - 1.19 3.21
- - 0.56 1.16
- - 0.46 1.49 0.53 1.64 0.96 2.73
- -
0.87 1.83
0.400
0.009
<0.001 0.008
0.250
- 0.525 0.805 0.080
0.215
Severity of aplastic anemia - SAA (ref)
- Very SAA
G-CSF randomization - No G-CSF (ref)
- With G-CSF
Age groups at randomization - < 20 years (ref)
- 20-39 years
- 40-59 years
- ≥60 years
Severity of aplastic anemia - SAA (ref)
- Very SAA
G-CSF:granulocytecolony-stimulatingfactor;SAA:severeaplastic anemia.
1228
haematologica | 2020; 105(5)