Page 69 - Haematologica May 2020
P. 69
Long-term outcome of ATG & CSA ± G-CSF in SAA
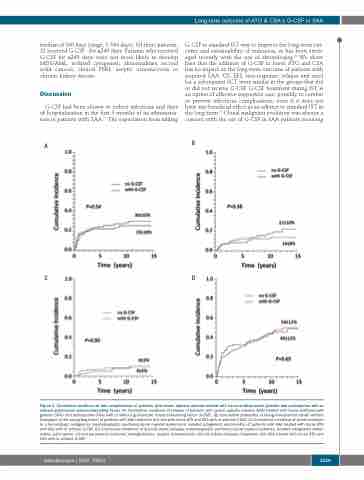
median of 160 days (range, 1-544 days). Of these patients, 21 received G-CSF for ≥240 days. Patients who received G-CSF for ≥240 days were not more likely to develop MDS/AML, isolated cytogenetic abnormalities, second solid cancers, clinical PNH, aseptic osteonecrosis or chronic kidney disease.
Discussion
G-CSF had been shown to reduce infections and days of hospitalization in the first 3 months of its administra- tion in patients with SAA.14 The expectation from adding
A
G-CSF to standard IST was to improve the long-term out- come and sustainability of remission, as has been envis- aged recently with the use of eltrombopag.24 We show here that the addition of G-CSF to horse ATG and CSA has no impact on the long-term outcome of patients with acquired SAA: OS, EFS, non-response, relapse and need for a subsequent SCT were similar in the groups that did or did not receive G-CSF. G-CSF treatment during IST is an option of effective supportive care, possibly to combat or prevent infectious complications, even if it does not have any beneficial effect as an adjunct to standard IST in the long-term.25 Clonal malignant evolution was always a concern with the use of G-CSF in SAA patients receiving
B
CD
Figure 3. Cumulative incidence of late complications of patients with severe aplastic anemia treated with horse antithymocyte globulin and cyclosporine with or without granulocyte colony-stimulating factor. (A) Cumulative incidence of relapse of patients with severe aplastic anemia (SAA) treated with horse antithymocyte globulin (ATG) and cyclosporine (CSA) with or without granulocyte colony-stimulating factor (G-CSF). (B) Cumulative probability of being transplanted (death without transplant is the competing event) of patients with SAA treated in first-line with horse ATG and CSA with or without G-CSF. (C) Cumulative incidence of clonal evolution to a hematologic malignancy (myelodysplastic syndrome/acute myeloid leukemia or isolated cytogenetic abnormality) of patients with SAA treated with horse ATG and CSA with or without G-CSF. (D) Cumulative incidence of any late event (relapse, myelodysplastic syndrome/acute myeloid leukemia, isolated cytogenetic abnor- mality, solid cancer, clinical paroxysmal nocturnal hemoglobinuria, aseptic osteonecrosis, chronic kidney disease) of patients with SAA treated with horse ATG and CSA with or without G-CSF.
haematologica | 2020; 105(5)
1229