Page 66 - Haematologica May 2020
P. 66
A. Tichelli et al.
Results
Overall survival and event-free survival
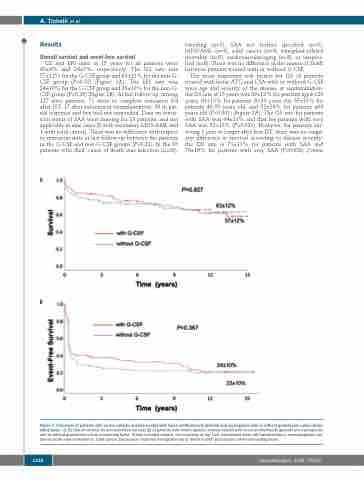
OS and EFS rates at 15 years for all patients were 60±9% and 24±7%, respectively. The OS rate was 57±12% for the G-CSF group and 63±12% for the non-G- CSF group (P=0.92) (Figure 1A). The EFS rate was 24±10% for the G-CSF group and 23±10% for the non-G- CSF group (P=0.36) (Figure 1B). At last follow-up, among 127 alive patients, 71 were in complete remission (54 after IST, 17 after subsequent transplantation), 29 in par- tial response and five had not responded. Data on remis- sion status of SAA were missing for 13 patients, and not applicable in nine cases (8 with secondary MDS/AML and 1 with solid cancer). There was no difference with respect to remission state at last follow-up between the patients in the G-CSF and non-G-CSF groups (P=0.81). In the 65 patients who died, cause of death was infection (n=26),
A
bleeding (n=3), SAA not further specified (n=3), MDS/AML (n=4), solid cancer (n=4), transplant-related mortality (n=8), cardiovascular/aging (n=9), or unspeci- fied (n=8). There was no difference in the causes of death between patients treated with or without G-CSF.
The most important risk factors for OS of patients treated with horse ATG and CSA with or without G-CSF were age and severity of the disease at randomization: the OS rate at 15 years was 89±12% for patients aged <20 years, 81±13% for patients 20-39 years old, 55±15% for patients 40-59 years old, and 32±16% for patients ≥60 years old (P<0.001) (Figure 2A). The OS rate for patients with SAA was 64±11% and that for patients with very SAA was 52±13% (P=0.021). However, for patients sur- viving 1 year or longer after first IST, there was no longer any difference in survival according to disease severity: the OS rate is 71±11% for patients with SAA and 74±16% for patients with very SAA (P=0.636) (Online
B
Figure 1. Outcomes of patients with severe aplastic anemia treated with horse antithymocyte globulin and cyclosporine with or without granulocyte colony-stimu- lating factor. (A, B) Overall survival (A) and event-free survival (B) of patients with severe aplastic anemia treated with horse antithymocyte globulin and cyclosporine with or without granulocyte colony-stimulating factor. Events included relapse, non-response at day 120, subsequent stem cell transplantation, myelodysplastic syn- drome/acute myeloid leukemia, solid cancer, paroxysmal nocturnal hemoglobinuria or death. G-CSF: granulocyte colony-stimulating factor.
1226
haematologica | 2020; 105(5)