Page 291 - Haematologica May 2020
P. 291
Modeling of FVIII activity, bleeds and covariates
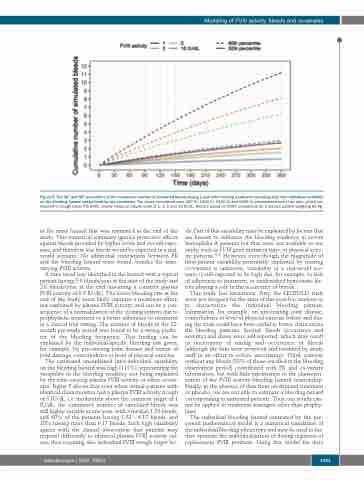
Figure 5. The 50th and 90th percentiles of the cumulative number of simulated bleeds during 1 year after starting treatment assuming only inter-individual variability on the bleeding hazard unexplained by any covariates. The doses considered were 420 IU, 1260 IU, 2100 IU and 6240 IU administered every two days, which cor- respond to trough factor VIII (FVIII) activity values at steady-state of 1, 3, 5 and 15 IU/dL. Results based on 2000 simulations for a median patient weighing 60 kg.
at the same hazard that was estimated at the end of the study. This numerical summary ignores protective effects against bleeds provided by higher levels and overall expo- sure, and therefore less bleeds would be expected in a real- world scenario. No additional correlations between PK and the bleeding hazard were found, besides the time- varying FVIII activity.
A time trend was identified in the hazard with a typical patient having 5.0 bleeds/year at the start of the study and 2.8 bleeds/year at the end (assuming a constant plasma FVIII activity of 0.5 IU/dL). The lower bleeding rate at the end of the study most likely captures a treatment effect, not explained by plasma FVIII activity, and can be a con- sequence of a normalization of the clotting system due to prophylactic treatment or a better adherence to treatment in a clinical trial setting. The number of bleeds in the 12- month pre-study period was found to be a strong predic- tor of the bleeding frequency. This finding can be explained by the individual-specific bleeding risk given, for example, by pre-existing joint disease and extent of joint damage, comorbidities or level of physical exercise.
The estimated unexplained inter-individual variability on the bleeding hazard was high (111%), representing the variability in the bleeding tendency not being explained by the time-varying plasma FVIII activity or other covari- ates. Figure 5 shows that even when virtual patients with identical characteristics had a plasma FVIII activity trough of 5 IU/dL, i.e. moderately above the common target of 1 IU/dL, the cumulative number of simulated bleeds was still highly variable at one year, with a median 1.53 bleeds, and 40% of the patients having 1.53 - 6.17 bleeds, and 10% having more than 6.17 bleeds. Such high variability agrees with the clinical observation that patients may respond differently to identical plasma FVIII activity val- ues, thus requiring also individual FVIII trough target lev-
els. Part of this variability may be explained by factors that are known to influence the bleeding tendency in severe hemophilia A patients but that were not available to our study, such as FVIII gene mutation type, or physical activ- ity patterns.45,46 However, even though the magnitude of inter-patient variability potentially explained by missing co-variates is unknown, variability in a real-world sce- nario is still expected to be high due, for example, to lack of adherence to treatment, or unidentified hemostatic fac- tors playing a role in the occurrence of bleeds.
This study has limitations. First, the LEOPOLD trials were not designed for the aims of this post-hoc analysis or to characterize the individual bleeding pattern. Information, for example, on pre-existing joint disease, comorbidities or level of physical exercise before and dur- ing the trials could have been useful to better characterize the bleeding patterns. Second, bleeds (occurrence and severity) and doses were self-reported, which may result in uncertainty of timing and occurrence of bleeds (although the data were reviewed and validated by study staff in an effort to reduce uncertainty). Third, patients without any bleeds (33% of those enrolled in the bleeding observation period) contributed with PK and co-variate information, but with little information to the characteri- zation of the FVIII activity-bleeding hazard relationship. Finally, in the absence of data from on-demand treatment or placebo, we are not able to estimate a bleeding hazard corresponding to untreated patients. Thus, our results can- not be applied to treatment strategies other than prophy- laxis.
The individual bleeding hazard estimated by the pre- sented mathematical model is a numerical translation of the individual bleeding phenotype and may be used to fur- ther optimize the individualization of dosing regimens of replacement FVIII products. Using this model for dose
haematologica | 2020; 105(5)
1451