Page 271 - Haematologica May 2020
P. 271
Different functions for FXII and PK in sepsis
release of antimicrobial peptides and bradykinin from HK, which trigger inflammatory reactions. On the other hand, activation of the system by the pathogen may provoke invasive spreading via bradykinin-induced vascular leak- age.5 We previously reported that S. pyogenes triggers acti- vation of the contact system by streptokinase with the lib- eration of bradykinin. In addition, we showed that S. pyo- genes isolates from invasive infections trigger an activa- tion of the contact system more potently than strains iso- lated from non-invasive infections. Intriguingly, no signif- icant difference was observed when plasmin activation was analyzed,23 supporting the idea that the ability of cer- tain strains to activate contact factors is associated with improved bacterial dissemination. The current study showed that PK, a serine protease of the contact system, is involved in fibrinolysis triggered by S. pyogenes, and supports streptococcal dissemination in mice. In vitro, degradation of fibrinogen and lysis of plasma clots (induced by streptokinase) was impaired in the absence of PK. We therefore, suggest that PK assist in degradation of fibrin by plasmin. This hypothesis is supported by ex vivo and in vivo experiments showing that PK is involved in S. pyogenes escape from mouse plasma clots and dissemi- nation. On the other hand, our data do not exclude a direct plasminogen activation by PK that was shown before in vitro.22 Of note, a knock-out of the Klkb1 gene in mice results in an antithrombotic phenotype.11,24 However, reduced thrombosis in Klkb1 KO mice was not due to defective contact activation but was a result of reduced aortic tissue factor in this mouse;24 thus, this mouse would be not suitable for our investigations. In the present study, we demonstrated that the selective reduction of PPK by
ASO-technology decelerated bacterial spreading, damp- ens inflammatory cytokine and chemokines, and raises CCL5 (RANTES). RANTES acts as a chemoattractant for monocytes, memory Th cells, and eosinophils. As in our animal model, in humans, the level of RANTES was inversely associated with bacteremia25 and the APACHE II score, thus low levels were predictive with poor outcome.26,27
FXII is the main physiological activator of PPK but, sur- prisingly, a knockdown of F12 gene had no influence on bacterial dissemination in our sepsis model. Beside its function as an activator for PPK, FXII directly increases the fiber density within a clot and makes it more resistant to fibrinolysis.28,29 Consequently, deficiency of FXII in mice or human impairs thrombus stability.30,31 The present study supports these findings, as fibrinolysis, initiated in vitro by bacteria or pure streptokinase, was faster in the absence of FXII. A recent study by Stroo et al. showed that FXII defi- ciency in mice improved survival and reduced bacterial outgrowth in an airway infection model with the Gram- negative Klebsiella pneumoniae. However, and similar to our data, FXII deficiency did not protect mice when the Gram-positive Streptococcus pneumoniae was used in the air- way infection model.32
A depletion of contact factors is often seen in sepsis patients,15,33,34 and this was confirmed in the present study. Depletion of contact factors has been assigned to con- sumption resulting from massive activation of the contact system. However, although a prolonged aPTT indicated a consumption of FXII and PK in these patients, we could not observe a massive HK cleavage.14 As contact factors are mainly synthesized in the liver, an alternative explana-
AB
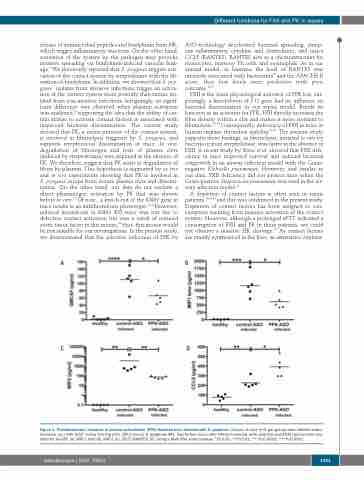
CD
Figure 4. Proinflammatory response in plasma prekallikrein (PPK)-depleted mice infected with S. pyogenes. Groups of mice (n=5 per group) were infected subcu- taneously (sc.) with 2x107 colony forming units (CFU)/mouse S. pyogenes AP1. Twenty-four hours after infection animals were collected and EDTA plasma were ana- lyzed for GmCSF (A), MIP-1 beta (B), MIP-2 (C), CCL5 (RANTES) (D), using a Multi-Plex immunoassay. *P≤0.05; **P≤0.01; ***P≤0.0002; ***P≤0.0001.
haematologica | 2020; 105(5)
1431