Page 109 - Haematologica May 2020
P. 109
BCR-FGFR1 regulated by dimerization and chaperonin Hsp90
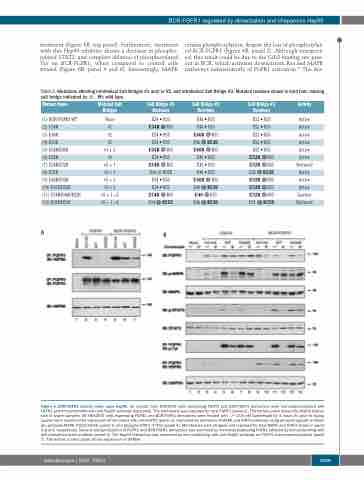
treatment (Figure 6B, top panel). Furthermore, treatment with this Hsp90 inhibitor shows a decrease in phospho- rylated STAT3, and complete ablation of phosphorylated Tyr on BCR-FGFR1, when compared to control cells treated (Figure 6B, panel 4 and 6). Interestingly, MAPK
retains phosphorylation, despite the loss of phosphorylat- ed BCR-FGFR1 (Figure 6B, panel 2). Although unexpect- ed, this result could be due to the Grb2 binding site pres- ent in BCR, which activates downstream Ras and MAPK pathways independently of FGFR1 activation.19 The dra-
Table 2. Mutations affecting interhelical Salt Bridges #1 and/or #2, and intrahelical Salt Bridge #3. Mutated residues shown in bold font; missing salt bridge indicated by ⨂ . Wt: wild-type.
Mutant Name
(1) BCR-FGFR1 WT
(2) E34R
(3) E46R
(4) R53E
(5) E34R/E46R
(6) E52R
(7) E34R/E52R
(8)R55E
(9) E46R/E52R
(10) R53E/E52R
(11) E34R/E46R/E52R
(12) R55E/R53E
A
Mutated Salt Bridges
None
#1
#2
#2
#1+2
#3
#3+1
Salt Bridge #1 Residues
E34 • R55
E34R ⨂ R55 E34 • R55 E34 • R55 E34R ⨂ R55 E34 • R55 E34R ⨂ R55
Salt Bridge #2 Residues
E46 • R53
E46 • R53 E46R ⨂ R53 E46 ⨂ R53E E46R ⨂ R53 E46 • R53 E46 • R53
Salt Bridge #3 Residues
E52 • R55
E52 • R55 E52 • R55 E52 • R55 E52 • R55 E52R ⨂ R55 E52R ⨂ R55 E52⨂R55E E52R ⨂ R55 E52R ⨂ R55 E52R ⨂ R55 E52 ⨂ R55E
Activity
Active
Active Active Active Active Active Reduced Active Active Active Inactive Reduced
#3+1 E34⨂R55E E46•R53
#3+2
#3+2
#3 + 1 +2
#3 + 1 +2
E34 • R55
E34 • R55
E34R ⨂ R55 E34 ⨂ R55E
B
E46R ⨂ R53 E46 ⨂ R53E E46 ⨂ R53 E46 ⨂ R53E
Figure 6. BCR-FGFR1 activity relies upon Hsp90. (A) Lysates from HEK293T cells expressing FGFR1 and BCR-FGFR1 derivatives were immunoprecipitated with FGFR1 and immunoblotted with anti-Hsp90 antibody (top panel). The membrane was reprobed for total FGFR1 (panel 2). The bottom panel shows the Hsp90 expres- sion in lysate samples. (B) HEK293T cells expressing FGFR1 and BCR-FGFR1 derivatives were treated with –/+ 200 nM Ganetespib for 4 hours (h) prior to lysing. Lysates were examined for expression of the clones with anti-FGFR1 (panel 1), examined for activation of MAPK and STAT3 pathways using phospho-specific antibod- ies; phospho-MAPK (T202/Y204) (panel 2) and phospho-STAT3 (Y705) (panel 4). Membranes were stripped and reprobed for total MAPK and STAT3 shown in panel 3 and 5, respectively. Tyrosine phosphorylation of FGFR1 and BCR-FGFR1 derivatives was examined by immunoprecipitating FGFR1 followed by immunoblotting with anti-phosphotyrosine antibody (panel 6). The Hsp90 interaction was examined by immunoblotting with anti-Hsp90 antibody on FGFR1 immunoprecipitations (panel 7). The bottom control panel shows expression of GAPDH.
haematologica | 2020; 105(5)
1269