Page 106 - Haematologica May 2020
P. 106
M.N. Peiris et al.
AD
BE
CF
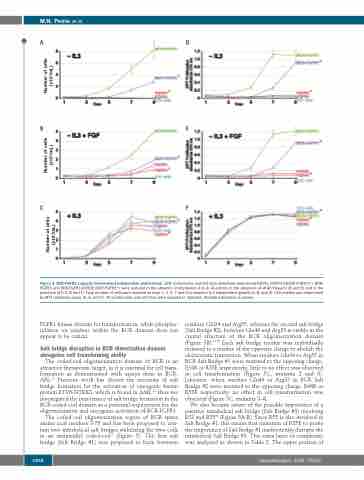
Figure 3. BCR-FGFR1 supports interleukin-3-independent proliferation. 32D control cells and cell lines selectively expressing FGFR1, FGFR1-K656E (FGFR1*), BCR- FGFR1 and BCR-FGFR1-K656E (BCR-FGFR1*) were cultured in the absence of interleukin-3 (IL-3) (A and D), in the presence of aFGF/Heparin (B and E) and in the presence of IL-3 (C and F). Total number of cells were counted on days 1, 3, 5, 7 and 9 to examine IL-3 independent growth (A, B, and C). Cell viability was determined by MTT metabolic assay (D, E, and F). All control cells and cell lines were assayed in triplicate. Standard deviation is shown.
FGFR1 kinase domain for transformation, while phospho- rylation on residues within the BCR domain does not appear to be critical.
Salt bridge disruption in BCR dimerization domain abrogates cell transforming ability
The coiled-coil oligomerization domain of BCR is an attractive therapeutic target, as it is essential for cell trans- formation as demonstrated with assays done in BCR- ABL.21 Previous work has shown the necessity of salt bridge formation for the activation of oncogenic fusion protein ETV6-NTRK3, which is found in AML.22 Here we investigated the importance of salt bridge formation in the BCR coiled-coil domain as a potential requirement for the oligomerization and oncogenic activation of BCR-FGFR1.
The coiled-coil oligomerization region of BCR spans amino acid residues 3-75 and has been proposed to con- tain two interhelical salt bridges stabilizing the two coils in an antiparallel coiled-coil23 (Figure 5). The first salt bridge (Salt Bridge #1) was proposed to form between
residues Glu34 and Arg55, whereas the second salt bridge (Salt Bridge #2), between Glu46 and Arg53 is visible in the crystal structure of the BCR oligomerization domain (Figure 5B).23-25 Each salt bridge residue was individually mutated to a residue of the opposite charge to abolish the electrostatic interaction. When residues Glu34 or Arg55 in BCR Salt Bridge #1 were mutated to the opposing charge, E34R or R55E respectively, little to no effect was observed in cell transformation (Figure 5C, mutants 2 and 8). Likewise, when residues Glu46 or Arg53 in BCR Salt Bridge #2 were mutated to the opposing charge, E46R or R53E respectively, no effect in cell transformation was observed (Figure 5C, mutants 3-4).
We also became aware of the possible importance of a putative intrahelical salt bridge (Salt Bridge #3) involving E52 and R5523 (Figure 5A-B). Since R55 is also involved in Salt Bridge #1, this means that mutation of R55E to probe the importance of Salt Bridge #1 inadvertently disrupts the intrahelical Salt Bridge #3. This extra layer of complexity was analyzed as shown in Table 2. The upper portion of
1266
haematologica | 2020; 105(5)
Number of cells Number of cells (x106/mL) (x105/mL) (x105/mL)
Number of cells