Page 68 - Haematologica April 2020
P. 68
P. Balogh et al.
and function. By immunoblot, the initial undifferentiated population displayed relatively high RUNX3 levels, with a gradual decline occurring during erythroid differentiation (Online Supplementary Figure S2A). Immunofluorescent staining revealed predominantly cytoplasmic localization in the undifferentiated cells and enhanced nuclear localiza- tion associated with erythroid differentiation (Online Supplementary Figure S3). Transduction of HSPC with empty or RUNX3-targeting lentiviral shRNA vectors did not alter the localization of RUNX3 in erythroid differen- tiated cells. Both nuclear and cytoplasmic patterns of RUNX3 localization have been observed in prior studies, and may reflect SMAD or STAT activation status as previ- ously described.29-32
Partial knockdown of RUNX3 with three independent lentiviral short RNA hairpins blocked erythroid differenti- ation of CD34+ progenitors, preventing expression of gly- cophorin A (CD235a) (Figure 2A and Online Supplementary Figure S2B). Subsequent experiments employed short hair- pin #4 due to robust knockdown (approx. 60% protein loss) with no significant cross-inhibition of other RUNX proteins (Online Supplementary Figure S2C). As additional controls, CD34+ progenitors also underwent transduction with shRNA vectors targeting GFP, which had no effect on erythroid differentiation, and RUNX1, which slightly enhanced erythroid differentiation as described33 (Online
Supplementary Figure S2D and E). RUNX3 deficiency in CD34+ HSPC also blocked erythroid colony formation in semi-solid medium, with no significant impact on mono- cyte or mixed granulocyte-monocyte colonies (Figure 2B). As with the colony assays, RUNX3 deficiency caused min- imal changes in granulocyte differentiation (CD15) after eight days of suspension culture (Figure 2C). When main- tained in uni-lineage, serum-free erythroid medium con- taining EPO and stem cell factor (SCF), RUNX3-deficient progenitors showed time-dependent declines in prolifera- tion and viability (Figure 2D and E). By contrast, RUNX3-deficient progenitors cultured in expansion medi- um with SCF, IL-3, thrombopoietin (TPO), and Flt3-ligand retained normal proliferation and near-normal viability (Figure 2D and E). However, RUNX3 knockdown did pre- vent HSPC upregulation of CD41 in megakaryocytic cul- tures, suggesting an influence at the level of erythro- megakaryocytic progenitors (Online Supplementary Figure S2F).
To determine contributions to post-commitment human erythropoiesis, we knocked down RUNX3 in sort- ed CD36+CD235a– early erythroid progenitors. RUNX3 deficiency in these cells impaired their progression to the more mature CD36+CD235a+ stage, indicating involve- ment in post-commitment differentiation (Figure 2F). Knockdown of RUNX3 in the human HUDEP-2 pro-ery-
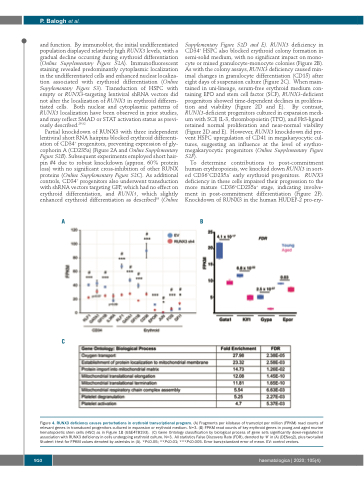
AB
C
Figure 4. RUNX3 deficiency causes perturbations in erythroid transcriptional program. (A) Fragments per kilobase of transcript per million (FPKM) read counts of relevant genes in transduced progenitors cultured in expansion or erythroid medium. N=3. (B) FPKM read counts of key erythroid genes in young and aged murine hematopoietic stem cells (HSC) as in Figure 1B (GSE478193). (C) Gene Ontology classification by biological process of gene sets significantly down-regulated in association with RUNX3 deficiency in cells undergoing erythroid culture. N=3. All statistics False Discovery Rate (FDR), denoted by ‘#’ in (A) (DESeq2), plus two-tailed Student t-test for FPKM values denoted by asterisks in (A). *P≤0.05; **P≤0.01; ***P≤0.005. Error bars±standard error of mean. EV: control vectors.
910
haematologica | 2020; 105(4)