Page 59 - Haematologica April 2020
P. 59
Megakaryocytes modulate BM cell mobilization
AB
CD
EFG
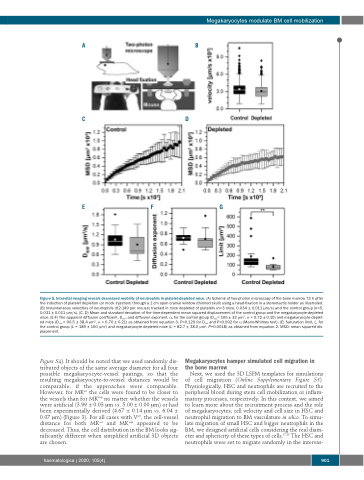
Figure 5. Intravital imaging reveals decreased mobility of neutrophils in platelet-depleted mice. (A) Scheme of two-photon microscopy of the bone marrow 72 h after the induction of platelet depletion (or mock injection) through a 1 cm open cranial window (thinned skull) using a head-fixation in a stereotactic holder as illustrated. (B) Instantaneous velocities of neutrophils (62-149 per mouse) tracked in mice depleted of platelets (n=3 mice, 0.034 ± 0.011 μm/s) and the control group (n=5, 0.031 ± 0.011 μm/s). (C, D) Mean and standard deviation of the time-dependent mean squared displacement of the control group and the megakaryocyte-depleted mice. (E-F) The apparent diffusion coefficient, Dapp, and diffusion exponent, α, for the control group (Dapp= 106 ± 32 μm2, α = 0.72 ± 0.10) and megakaryocyte-deplet- ed mice (Dapp = 90.5 ± 38.4 μm2, α = 0.70 ± 0.21) as obtained from equation 3. P=0.129 for Dapp and P=0.092 for α (Mann-Whitney test). (E) Saturation limit, L, for the control group (L = 189 ± 150 μm2) and megakaryocyte-depleted mice (L = 82.7 ± 38.0 μm2, P=0.0018) as obtained from equation 2. MSD: mean squared dis- placement.
Figure S4). It should be noted that we used randomly dis- tributed objects of the same average diameter for all four possible megakaryocyte-vessel pairings, so that the resulting megakaryocyte-to-vessel distances would be comparable, if the approaches were comparable. However, for MKart the cells were found to be closer to the vessels than for MKexp no matter whether the vessels were artificial (3.99 ± 0.03 μm vs. 5.00 ± 0.00 μm) or had been experimentally derived (4.67 ± 0.14 μm vs. 6.04 ± 0.07 μm) (Figure 3). For all cases with Vart, the cell-vessel distance for both MKart and MKexp appeared to be decreased. Thus, the cell distribution in the BM looks sig- nificantly different when simplified artificial 3D objects are chosen.
Megakaryocytes hamper simulated cell migration in the bone marrow
Next, we used the 3D LSFM templates for simulations of cell migration (Online Supplementary Figure S5). Physiologically, HSC and neutrophils are recruited to the peripheral blood during stem cell mobilization or inflam- matory processes, respectively. In this context, we aimed to learn more about the recruitment process and the role of megakaryocytes, cell velocity and cell size in HSC and neutrophil migration to BM vasculature in silico. To simu- late migration of small HSC and bigger neutrophils in the BM, we designed artificial cells considering the real diam- eter and sphericity of these types of cells.37,38 The HSC and neutrophils were set to migrate randomly in the intervas-
haematologica | 2020; 105(4)
901