Page 317 - Haematologica April 2020
P. 317
Evaluating Netherlands’ transition from FFP to SD plasma
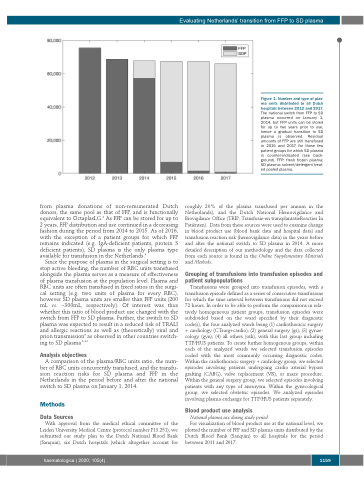
Figure 1. Number and type of plas- ma units distributed to all Dutch hospitals between 2012 and 2017. The national switch from FFP to SD plasma occurred on January 1, 2014, but FFP units can be stored for up to two years prior to use, hence a gradual transition to SD plasma is observed. Residual amounts of FFP are still transfused in 2016 and 2017 for those few patient groups for which SD plasma is counter-indicated (see back- ground). FFP: fresh frozen plasma; SD plasma: solvent/detergent treat- ed pooled plasma.
from plasma donations of non-remunerated Dutch donors, the same pool as that of FFP, and is functionally equivalent to OctaplasLG.3 As FFP can be stored for up to 2 years, FFP distribution and use continued in a decreasing fashion during the period from 2014 to 2015. As of 2016, with the exception of a patient groups for which FFP remains indicated (e.g. IgA-deficient patients, protein S deficient patients), SD plasma is the only plasma type available for transfusion in the Netherlands.4
Since the purpose of plasma in the surgical setting is to stop active bleeding, the number of RBC units transfused alongside the plasma serves as a measure of effectiveness of plasma transfusion at the population level. Plasma and RBC units are often transfused in fixed ratios in the surgi- cal setting (e.g. two units of plasma for every RBC), however SD plasma units are smaller than FFP units (200 mL vs. ~300mL, respectively). Of interest was thus whether this ratio of blood product use changed with the switch from FFP to SD plasma. Further, the switch to SD plasma was expected to result in a reduced risk of TRALI and allergic reactions as well as (theoretically) viral and prion transmission5 as observed in other countries switch- ing to SD plasma.6–14
Analysis objectives
A comparison of the plasma/RBC units ratio, the num- ber of RBC units concurrently transfused, and the transfu- sion reaction risks for SD plasma and FFP in the Netherlands in the period before and after the national switch to SD plasma on January 1, 2014.
Methods
Data Sources
With approval from the medical ethical committee of the Leiden University Medical Centre (protocol number P13.251), we submitted our study plan to the Dutch National Blood Bank (Sanquin), six Dutch hospitals (which altogether account for
roughly 20% of the plasma transfused per annum in the Netherlands), and the Dutch National Hemovigilance and Biovigilance Office (TRIP: Transfusie-en transplantatieReacties In Patiënten). Data from these sources were used to examine change in blood product use (blood bank data and hospital data) and transfusion reaction risk (hemovigilance data) in the years before and after the national switch to SD plasma in 2014. A more detailed description of our methodology and the data collected from each source is found in the Online Supplementary Materials and Methods.
Grouping of transfusions into transfusion episodes and patient subpopulations
Transfusions were grouped into transfusion episodes, with a transfusion episode defined as a series of consecutive transfusions for which the time interval between transfusions did not exceed 72 hours. In order to be able to perform the comparisons in rela- tively homogeneous patient groups, transfusion episodes were subdivided based on the ward specified by their diagnostic code(s), the four analyzed wards being (1) cardiothoracic surgery + cardiology (CTsurg+cardio); (2) general surgery (gs); (3) gynae- cology (gyn); (4) all others (oth), with this last group including TTP/HUS patients. To create further homogenous groups, within each of the analyzed wards we selected transfusion episodes coded with the most commonly occurring diagnostic codes. Within the cardiothoracic surgery + cardiology group, we selected episodes involving patients undergoing cardio arterial bypass grafting (CABG), valve replacement (VR), or maze procedure. Within the general surgery group, we selected episodes involving patients with any type of aneurysm. Within the gynecological group, we selected obstetric episodes. We analyzed episodes involving plasma exchange for TTP/HUS patients separately.
Blood product use analysis
National plasma use during study period
For visualization of blood product use at the national level, we plotted the number of FFP and SD plasma units distributed by the Dutch Blood Bank (Sanquin) to all hospitals for the period between 2011 and 2017.
haematologica | 2020; 105(4)
1159