Page 292 - Haematologica April 2020
P. 292
J. Russick et al.
h, prophylactic replacement therapy is the gold standard to maintain healthy joint function.37 In FVIII-deficient mice, the half-life of human FVIII is between 4 and 6 h. Interestingly, delivery of FVIII-encoding mRNA yielded FVIII levels that corrected acute bleeding in a tail clipping assay, and were maintained above 5% for up to 72 h. The longer residence time of FVIII produced after mRNA delivery as compared to that of rFVIII results from the cumulative lifespans of the transfected mRNA in the cells and of the FVIII released in the circulation. mRNA-based therapy can be improved by engineering the encoded protein to extend its half-life.38 For instance, a FVIII mole- cule with an increased half-life has been developed by fusion of FVIII to the Fc fragment of human IgG1.48 We anticipate that the use of mRNA encoding long-lasting FVIII glycoproteins will allow a further increase in the duration of FVIII detection in vivo. Because peak expres- sion levels of FVIII were reached in the mice between 6 and 24 h after the administration of mRNA, mRNA-based therapy may represent a surrogate for prophylactic treat- ment rather than for the on-demand treatment of sudden acute hemorrhagic events.
The specific activity of the rFVIII produced by eukaryot- ic cells in vitro was about 1.2 following transfection using cDNA and about 0.7 following transfection with mRNA. In contrast, the specific activity of the FVIII produced endogenously following injection of mRNA to the mice was about 0.3, suggesting the absence of pro-coagulant activity for a substantial proportion of the endogenously produced FVIII molecules. In parallel, we compared the levels of intact FVIII measured using a standard ELISA, wherein FVIII is captured with an anti-light chain anti- body and detected with anti-heavy chain antibody, with that of FVIII light chain measured using a sandwich light chain-specific ELISA. A perfect correlation was obtained although with an overall tendency for 2-fold more light chains than intact molecules. Importantly, the levels of light chain may be under-estimated because of the short in vivo half-life of the light chain alone. Future experiments will indicate whether the use of mRNA encoding FVIII lacking the furin cleavage site39,40 between the heavy and light chains, or FVIII mutants with increased FVIII A2 sub- unit stability41 yields FVIII with improved specific activity. An alternative and non-exclusive explanation for the poor specific activity of the endogenously produced FVIII is the fact that the TransIT® used to formulate the mRNA does not target particular cell types. In fact, we show here that luciferase is produced by the spleen, rather than by the liver, following injection of mice with luciferase-encoding mRNA formulated with TransIT®. Accordingly, immuno- fluorescence experiments on mice treated with FVIII- encoding mRNA occasionally detected faint amounts of FVIII in the marginal zone of the spleen but never in the liver (not shown). Hence, TransIT® delivers FVIII mRNA to cells that are not dedicated to the production of FVIII,42,43 thus potentially generating FVIII molecules that are improperly folded or have undergone incorrect post-trans- lational modifications.
In patients with severe hemophilia A, inhibitory anti- FVIII IgG (FVIII inhibitors) generally develop within the first 20 cumulated days of exposure to therapeutic FVIII.44 In the present work, and in agreement with previous stud- ies in FVIII-deficient mice,45 FVIII-binding IgG and FVIII inhibitors were detected after two and three intravenous injections of rFVIII, respectively. The levels of anti-FVIII
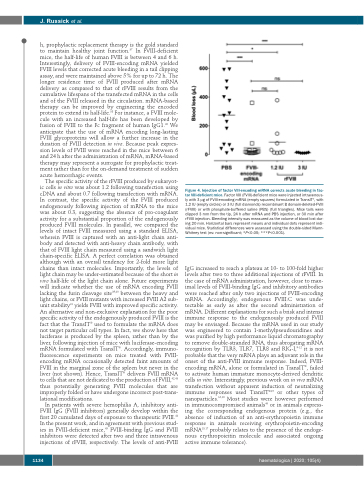
Figure 4. Injection of factor VIII-encoding mRNA corrects acute bleeding in fac- tor VIII-deficient mice. Factor VIII (FVIII)-deficient mice were injected intravenous- ly with 3 μg of FVIII-encoding mRNA (empty squares) formulated in TransIT®, with 1.2 IU (empty circles) or 3 IU (full diamonds) recombinant B domain-deleted-FVIII (rFVIII) or with phosphate-buffered saline (PBS) (full triangles). Mice tails were clipped 3 mm from the tip, 24 h after mRNA and PBS injection, or 30 min after rFVIII injection. Bleeding intensity was measured as the volume of blood lost dur- ing 20 min. Horizontal bars represent means and individual dots represent indi- vidual mice. Statistical differences were assessed using the double-sided Mann- Whitney test (ns: non-significant; *P<0.05; ***P<0.001).
IgG increased to reach a plateau at 10- to 100-fold higher levels after two to three additional injections of rFVIII. In the case of mRNA administration, however, close to max- imal levels of FVIII-binding IgG and inhibitory antibodies were reached after only two injections of FVIII-encoding mRNA. Accordingly, endogenous FVIII:C was unde- tectable as early as after the second administration of mRNA. Different explanations for such a brisk and intense immune response to the endogenously produced FVIII may be envisaged. Because the mRNA used in our study was engineered to contain 1-methylpseudouridines and was purified by high performance liquid chromatography to remove double-stranded RNA, thus abrogating mRNA recognition by TLR3, TLR7, TLR8 and RIG-I,9-12 it is not probable that the very mRNA plays an adjuvant role in the onset of the anti-FVIII immune response. Indeed, FVIII- encoding mRNA, alone or formulated in TransIT®, failed to activate human immature monocyte-derived dendritic cells in vitro. Interestingly, previous work on in vivo mRNA transfection without apparent induction of neutralizing immune responses used TransIT®15 or other types of nanoparticles.16,18 Most studies were however performed in immunocompromised animals46 or in animals express- ing the corresponding endogenous protein (e.g., the absence of induction of an anti-erythropoietin immune response in animals receiving erythropoietin-encoding mRNA15,17 probably relates to the presence of the endoge- nous erythropoieitin molecule and associated ongoing active immune tolerance).
1134
haematologica | 2020; 105(4)