Page 293 - Haematologica April 2020
P. 293
mRNA-based therapy for hemophilia A
AB
CD
EF
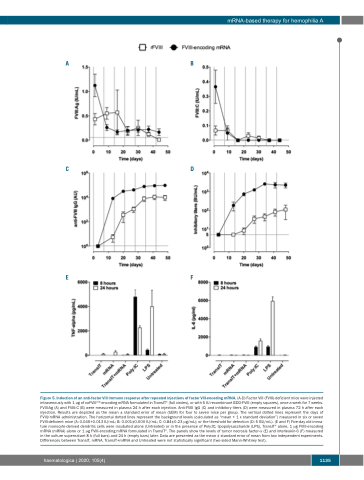
Figure 5. Induction of an anti-factor VIII immune response after repeated injections of factor VIII-encoding mRNA. (A-D) Factor VIII (FVIII)-deficient mice were injected intravenously with 1 μg of coFVIIIHSQ-encoding mRNA formulated in TransIT® (full circles), or with 5 IU recombinant BDD-FVIII (empty squares), once a week for 7 weeks. FVIII:Ag (A) and FVIII:C (B) were measured in plasma 24 h after each injection. Anti-FVIII IgG (C) and inhibitory titers (D) were measured in plasma 72 h after each injection. Results are depicted as the mean ± standard error of mean (SEM) for four to seven mice per group. The vertical dotted lines represent the days of FVIII/mRNA administration. The horizontal dotted lines represent the background levels (calculated as “mean + 1 x standard deviation”) measured in six or seven FVIII-deficient mice (A: 0.045+0.013 IU/mL; B: 0.001±0.000 IU/mL; C: 0.84±0.23 μg/mL); or the threshold for detection (D: 5 BU/mL). (E and F) Five-day old imma- ture monocyte-derived dendritic cells were incubated alone (Untreated) or in the presence of Poly:IC, lipopolysaccharide (LPS), TransIT® alone, 1 μg FVIII-encoding mRNA (mRNA) alone or 1 μg FVIII-encoding mRNA formulated in TransIT®. The panels show the levels of tumor necrosis factor-α (E) and interleukin-6 (F) measured in the culture supernatant 8 h (full bars) and 24 h (empty bars) later. Data are presented as the mean ± standard error of mean from two independent experiments. Differences between TransIT, mRNA, TransIT+mRNA and Untreated were not statistically significant (two-sided Mann-Whitney test).
haematologica | 2020; 105(4)
1135